Axo-axonal interactions of neuronal cells play an important role in functional development during embryogenesis. Axons of the cells formed on early stages of the brain development provide a template for the growing axons of later axons. However, the mechanisms of the guiding of younger axons by already formed axons are not well understood. In this study, we present a method to study such axo-axonal interactions in vitro using microfluidics methods and culturing neocortical cells. We studied the dynamics of axon growth in microchannels perpendicularly intersecting with other microchannels. This study provides fundamental understanding of the axonal navigation in microfluidic structures, which further facilitate the design of experimental in vitro model for studying the role of already formed axons in the development of neuronal system.
Introduction
The formation of neural circuits in neurogenesis and during brain development is a one of the key problems of neurobiology. Disorders of axon navigation lead to the formation of an abnormal network structure and brain dysfunction, i.e. the absence or underdevelopment of the corpus callosum, L1 syndrome, Joubert syndrome, Kallmann syndrome and other disorders (Engle, 2010). The growth and navigation of axons during the development of the nervous system is a complex multistep process occurring under conditions of various types of structural and chemical anisotropy.
The interaction of growing axons with already formed network of other cells is an important process for proper connectivity formation in the brain. The processes of neighboring cells are used by neurons as a guidance for migration and axon navigation. In particular, during neocortex development, the processes of radial glial cells are used for migration of neurons to the upper layers. It is known that axons formed in early stage of the development influence axonal navigation of later growing neurons (Eisen et al., 1986; Wang and Marquardt, 2013; Zhou et al., 2013; Cioni et al., 2018). For example, axo-axonal interactions form optic tract (Cioni et al., 2018) and the motor neuron connectivity (Eisen et al., 1986). Early formed projections of Cajal-Retzius cells from Dentate gyrus to entorhinal cortex provide a template for outgrowing entorhinal axons (Ceranik et al., 1999). In these case, the axons of new differentiated neurons interact with already grown axons and then grow predominantly in one direction: proximally or distally from the soma of the "guiding" axons.
Despite numerous studies of the navigation of neuronal cells processes, the mechanisms of such interactions during axon navigation are still not fully understood (Arlotta et al., 2005; Fame et al., 2011; Greig et al., 2013; Lodato et al., 2015). In particular, the mechanisms of axonal navigation are poorly understood in the early stages of development of the corpus callosum - the main commissure of the brain is necessary for highly specialized functions, such as speech processing (Josse et al., 2008; Hinkley et al., 2016). Experimental study of the axo-axonal interactions in vivo is exceptionally a difficult task due to the complexity of the brain organization and the continuous modulation of axonal growth by multiple chemical and physical factors of the environment.
Application of novel microfluidics methods to the neuronal networks in vitro allows to isolate neuronal processes from the cell bodies and guide them in a desired direction in order to perform biochemical or electrophysiological analysis of isolated axons (Pan et al., 2011; Siddique and Thakor, 2014; Yap and Dickson, 2014; Le Feber et al., 2015; Habibey et al., 2015a; Habibey et al., 2015b; Poli et al., 2018; Renault et al., 2015; Roth et al., 2012; Taylor et al., 2010, 2015).
In this study, we proposed a new method to mimic axo-axonal interactions in vitro in order to uncover mechanisms of axon navigation in such conditions. We developed a microfluidic chip which consisted of two chambers with separate cultures. One chamber had two microchannels for “guiding” axons (guiding microchannels) and other chamber had ten perpendicular lateral channels for the “guided”axons. The microchannels had T-shape intersection points where axons from two cultures interacted and “guided” axons had to change the growth direction either to a soma of “guiding” axons or opposite. We analyzed an efficiency of the method to obtain axon growth direction in several types of intersections and discussed further application of the method.
Materials and methods
Microfluidic chips
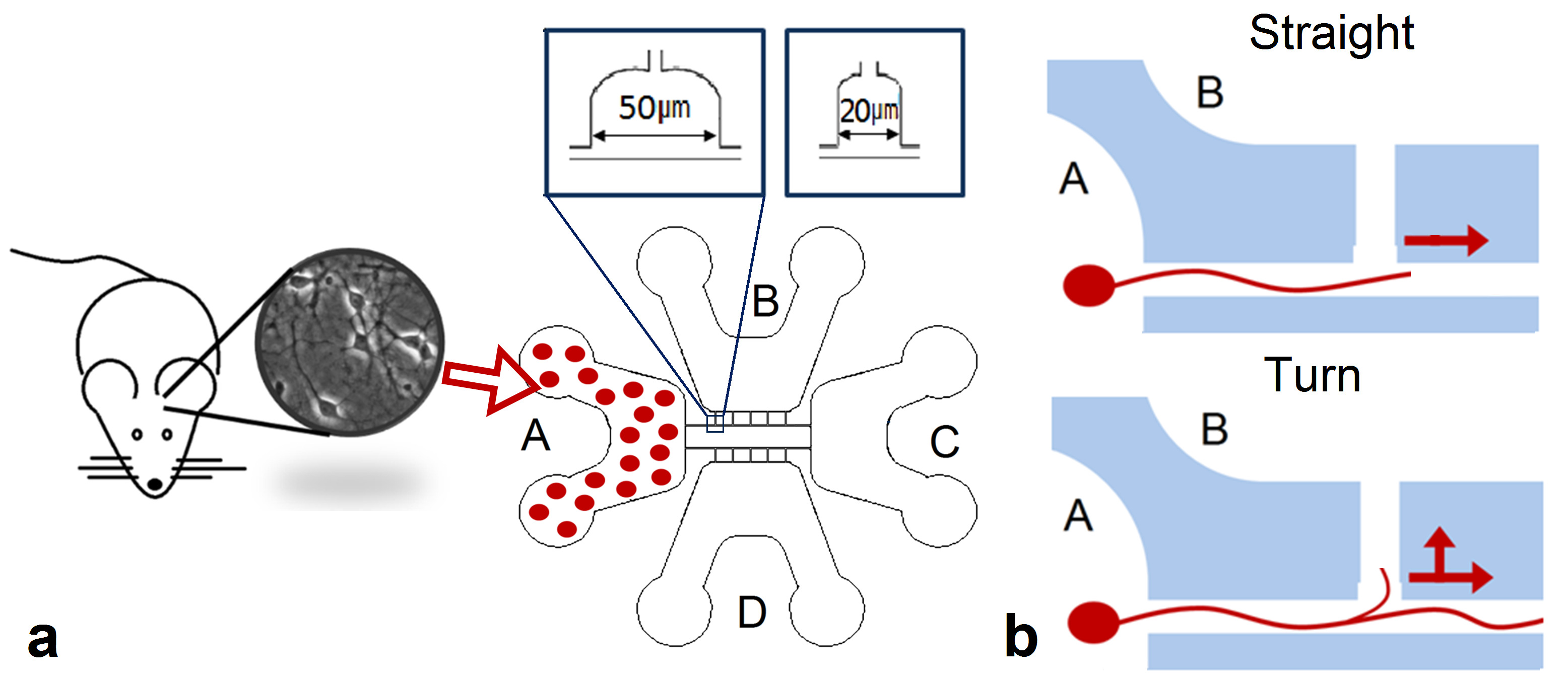
Microfluidic chips were made from biocompatible polydimethylsiloxane silicone (PDMS) using method of soft lithography (Fig. 1.a). A detailed description of the chip manufacturing method was explained in our early publication (Gladkov et al., 2017). The designed chip consisted of 4 chambers for cell cultures connected by microchannels. The height of the chambers for cell culture was equal to 200 μm. Round holes were punched in two opposite edges of the chambers to load a cell suspension.The height of the microchannels (3 μm) prevented to enter the cell soma but was sufficient to grow the neurities.
We used chips with a T-shaped configuration of the microchannels. The chips consisted of four chambers (Fig. 1 b, c). Two chambers (A and C) were located in opposite edges of the chip and were connected by two long straight microchannels 10 μm wide (guiding microchannels). Lateral chambers (B or D) were coupled with long microchannels with five lateral microchannels of 200 μm length and formed T-shaped intersections (10 T-shaped intersections in one chip). Extensions with widths of 20 and 50 μm in the area of intersections were made for imaging of the process of axon navigation during interaction with “guiding” axon (fig. 2 a). The first intersection with the lateral microchannel was located at a distance of 566 μm from chamber A. The distance between the intersections was 141 µm.
The surfaces of the prepared PDMS chips were bonded to a cover glass (24 * 24 mm). The glasses were placed on a custom designed 6-well plate (see Gladkov et al.,2017 for more details).The PDMS chips coated with polyethyleneimine (1 μg / ml; Sigma P3143) for 80 minutes at 37 ° C for adhesion.
Fig 1. Microfluidic chips a. Microfluidic chip fabrication steps. b. Microfluidic chip design. c. Microfluidic chip combined with a cover glass. Scale 5mm. d. Design of the plate with the chips.
Cortical cultures
Neocortical cells were obtained from mouse embryos (E17-E18). The basic rules for the maintenance and care of experimental animals complied with the standards given in the manual «Guide for the Care and Use of Laboratory Animals» (ILAR publication, 1996, The National Academies Press), in the National Standard of the Russian Federation - GOST 33044-2014 «Principles of Good Laboratory Practice (GLP)», and were agreed with the Ethics Committee.
The protocol of preparation and culturing of neuronal cells was similar to previous work (Gladkov et al., 2017). After plating, the cells were stored in a humidified CO2 incubator with 5% CO2 in the air. The plating medium completely covered the chip and consisted of a neurobasal medium (NBM, Invitrogen 21103±049) with 2% B27 (Invitrogen 17504±044), 0.5% l-glutamine (Invitrogen 25030±024) and 5% fetal calf serum (PanEco 055). After 24 hrs, a half of the plating medium was replaced with a culturing medium containing NBM, 2% B-27 and 1% glutamine and 0.5% fetal calf serum. Half of the medium was replaced 2-3 times a week.
Cells were plated only in the chamber A of T-chips to analyze axon navigation in microchannels while passing through the T-shaped intersections. The scheme of the experiment is shown in Figure 2. To study axonal interactions at the intersection of microchannels, it is necessary that axons from chamber A grow along the channel to chamber C without turning into lateral channels. Axonal navigation was analyzed in microfluidic chips of two configurations. The first type contained intersections 20 µm wide, the second - 50 µm (fig. 2 a).
Fig 2. Scheme of experiment. a. The scheme of plating cells in chamier A for evaluating axon trajectory during the growth through a T-shaped intersection.
Axon growth analysis
Neurite outgrowth dynamics was analysed with a system for the continuous monitoring of living cells in vitro (Cell IQ, ChipMan Technologies, Finland). The automatic system was used to monitor morphological dynamics of several cultures in the multi-well plates simultaneously in different regions. We started to monitor neurite growth 24 h after plating. The Cell IQ system continuously acquired phase contrast images with a ×20 objective (Nikon CFI Plan Fluorescence ELWD ADL, Japan) at interval of 20 min for each selected region. Data were processed and analysed with a Cell IQ Analyzer program.
In experiment 1 (by determining the effective shape of the intersection), we first recorded the time when the first axon grew into a long guiding microchannel. Then we recorded the time during which the axon grew in the microchannel up to each of the T-shaped intersections. Next, we recorded the time intervals from axonal growth to the guiding microchannel to its growth through the intersection in the forward direction (T straight) and its growth in the lateral microchannel (T turn). Schemes of axon growth in the forward direction and turning into the lateral microchannel are shown in Figure 2b.
The probability of axon to grow in the lateral microchannel (Pt) was calculated at various time intervals after the growth of the axon to the intersection (ΔT = T intersection - T turn). Pt was calculated as follows:
Pt = n (ΔT) / N * 100%,
where n(ΔT) is the number of intersections in which axon growth in the lateral microchannel at a certain time interval after axon grew to the intersection (ΔT); N is the total number of observed intersections. Pt indicated the effectiveness of the intersection to provide forward axonal growth. N was 5 intersections when Pt was calculated for one experiment (one guiding microchannel). In some cases, the guiding axon did not grow to the last intersection, or the channel was partially blocked with debris. In such cases, N was less than 5.
Statistical analysis
Statistical difference was performed with Mann-Whitney test to compare two independent samples and the Wilcoxon test to compare two related samples.. P-value < 0.05 (∗) was considered as the significant difference.
Results
Axonal growth in a T-shaped intersection
The cultured neurons, within 2-4 h after plating, started to sprout neurites into the guiding microchannels. The axons grew to the first intersection at a distance of 566 μm from chamber A within 1–7 days from the moment of growth into the guiding microchannel or within 3–10 days from the day of cell plating. On average, the time of axonal growth until reaching each subsequent intersection increased by 1 day.
In the area of intersection, the axons first grew in the forward direction or both: in the forward direction and into the lateral microchannel within one day. In rare cases, the axons could grow in the lateral microchannel without growing in the forward direction. The time of axonal growth in the forward direction was highly variable in different guiding microchannels.
Examples of the time of axonal growth through intersections in the forward direction (Tstraight) and into the lateral microchannel (Tturn) are shown in Fig. 3 a and с for intersections 50 and 20 μm wide, respectively. The average values of Tstraight and Tturn for intersections 50 µm (n = 5 guiding microchannels) and 20 µm wide (n = 9 guiding microchannels) are shown in Figure 3 b and d, respectively. Tstraight was significantly shorter than the Tturn at 1st and 2nd intersections with a width of 20 μm.
Fig. 3. Axonal growth through T-shaped intersections 50 and 20 μm wide in the forward direction and into the lateral microchannel. Upper diagram: Time of axonal growth through intersections in the forward direction (Tstraight) and into the lateral microchannel (Tturn) for intersections 50 μm wide. а. Example of one microchannel. b. Mean ± standard deviation (n = 5 guiding microchannels). Lower diagram: Time of axonal growth through intersections in the forward direction (Tstraight) and into the lateral microchannel (Tturn) for intersections 20 μm wide. c. Example of one microchannel. d. Mean ± standard deviation (n = 9 guiding microchannels). The differences between Tstraight and Tturn were analyzed with the Wilcoxon test, * p < 0.05.
Next step was to compare the growth of the axons at intersections of different widths 50 and 20 µm. For this, we analyzed time intervals between the growth of the axon to the intersection and growth in the lateral microchannel (ΔT, see methods). ΔT was calculated separately for each intersections from 1st to 5th. ΔT in 20 µm intersection was significantly longer than in 50 µm intersection only in second intersections (Fig. 3 a).
For wide 50 µm intersections, the probability of axonal growth in the lateral microchannel during the day from the moment of reaching the intersection (ΔT from 0 to 1 day) was 70.6% (n = 17 intersections). The axons did not grow in the lateral microchannel for more than 5 days after reaching the intersection in only one observed intersection (Pt = 5.9%, ΔT> 5 days).
For narrow 20 µm intersections, the probability of axonal growth in the lateral microchannel during a day from the moment of reaching the intersection (ΔT from 0 to 1 day) was 48,8% (n = 41 intersections), which is almost 1.5 times less than for wide intersections. The axons did not grow in the lateral microchannel for more than 5 days after reaching the intersection in 19,5% of observed intersections (Pt = 19.5%, ΔT> 5 days). The probability of axonal growth in the lateral microchannel after different ΔT is presented in Fig. 3c and f for intersections 50 and 20 μm wide, respectively. However, significant difference of Pt was not found (Fig. 3 d).
Fig. 4. Axonal growth in intersections with size of 50 and 20 µm. a. Time intervals between the growth of the axon to the intersection and growth in the lateral microchannel (ΔT, see methods) in 1-5 intersections with widths of 50 and 20 µm. The differences between ΔT in 50 and 20 µm intersections were analyzed with the Mann-Whitney test, * p < 0.05. b. The probability of axon growth in the lateral microchannel (Pt) at various time intervals after axon growth to the intersection (ΔT), n = 17 intersections. c. The probability of axon growth in the lateral microchannel (Pt) at various time intervals after axon growth to the intersection (ΔT), n = 41 intersections. d. Median ± 25th/75th percentile of the probability of axon growth in the lateral microchannel (Pt) during a day (T≤1). Mann-Whitney test, * p < 0.05.
Discussion
In this study we investigated only axon growth dynamics of the “guiding” axons from one population which should grow only in forward direction when it passes T-shaped intersections. We showed the navigation of “guiding” axons during the growth through the intersections was mostly forward but then within one day the axons grew in lateral channel. The result suggest plating of second population one day after the first population in order to obtain desired interaction of “guided” and “guiding” axons.
A temporary block of axon growth from chamber A to the lateral microchannel can increase the effectiveness of this experimental model in the future. This can be implemented, for example, using electrical stimulation and a collagen matrix (Honegger et al., 2013, 2016).
In this work, difference in the probability of axon growth in the lateral microchannel at the intersections of 50 and 20 μm was not significant. This could be due to large data variability. Nevertheless, a difference was found in the time intervals during which the axons growth only to the forward direction, after passing the intersections of 50 and 20 μm. The difference was found in the second intersection of microchannels at a distance of 707 μm from chamber A with cultured cells. These results indicate that narrow microchannels were more suitable for the axon grow only in the forward direction. Therefore, we plan to use narrow channels in works on the further development of the method of the intersection of individual axons.
Conclusion
We proposed a concept to study axo-axonal interaction of the neurons growing from two cellular populations in vitro. Axons grow in perpendicular microchannels until it cross intersection where further axon behaviour can be analyzed in real-time. The proposed approach can be used to study fundamental mechanisms of neocortex development, in particular the formation of the corpus callosum.
| Attachment | Size |
|---|---|
| 21.78 MB |
The reported study was funded by RFBR according to the research project № 18-34-00639.
Arlotta, P., Molyneaux, B. J., Chen, J., Inoue, J., Kominami, R., and MacKlis, J. D. (2005). Neuronal subtype-specific genes that control corticospinal motor neuron development in vivo. Neuron 45, 207–221. doi:10.1016/j.neuron.2004.12.036.
Ceranik, K., Deng, J., Heimrich, B., Lübke, J., Zhao, S., Förster, E., et al. (1999). Hippocampal Cajal-Retzius cells project to the entorhinal cortex: retrograde tracing and intracellular labelling studies. Eur. J. Neurosci. 11, 4278–4290. doi:10.1046/j.1460-9568.1999.00860.x.
Cioni, J. M., Wong, H. H. W., Bressan, D., Kodama, L., Harris, W. A., and Holt, C. E. (2018). Axon-Axon Interactions Regulate Topographic Optic Tract Sorting via CYFIP2-Dependent WAVE Complex Function. Neuron 97, 1078–1093.e6. doi:10.1016/j.neuron.2018.01.027.
Eisen, J. S., Myers, P. Z., and Westerfield, M. (1986). Pathway selection by growth cones of identified motoneurones in live zebra fish embryos. Nature 320, 269–271. doi:10.1038/320269a0.
Engle, E. C. (2010). Human disorders of axon guidance. Curr. Opin. Neurobiol. 22, 837–843. doi:10.1016/j.conb.2012.02.006.
Fame, R. M., MacDonald, J. L., and Macklis, J. D. (2011). Development, specification, and diversity of callosal projection neurons. Trends Neurosci. 34, 41–50. doi:10.1016/j.tins.2010.10.002.
Gladkov, A., Pigareva, Y., Kutyina, D., Kolpakov, V., Bukatin, A., Mukhina, I., et al. (2017). Design of Cultured Neuron Networks in vitro with Predefined Connectivity Using Asymmetric Microfluidic Channels. Sci. Rep., 1–14. doi:10.1038/s41598-017-15506-2.
Greig, L. C., Woodworth, M. B., Galazo, M. J., Padmanabhan, H., and Macklis, J. D. (2013). Molecular logic of neocortical projection neuron specification, development and diversity. Nat. Rev. Neurosci. 14, 755–769. doi:10.1038/nrn3586.
Habibey, R., Golabchi, A., and Blau, A. (2015a). Microchannel Scaffolds for Neural Signal Acquisition and Analysis. Neurotechnology, Electron. Informatics, Springer Ser. Comput. Neurosci. 13, 47–64. doi:10.1007/978-3-319-15997-3_4.
Habibey, R., Golabchi, A., Latifi, S., Difato, F., and Blau, A. (2015b). Microchannel device for selective laser dissection, long-term microelectrode array electrophysiology and imaging of confined axonal projections. Lab Chip 15, 4578–4590. doi:10.1039/C5LC01027F.
Hinkley, L. B. N., Marco, E. J., Brown, E. G., Bukshpun, P., Gold, J., Hill, S., et al. (2016). The Contribution of the Corpus Callosum to Language Lateralization. J. Neurosci. 36, 4522–4533. doi:10.1523/jneurosci.3850-14.2016.
Honegger, T., Scott, M. A., Yanik, M. F., and Voldman, J. (2013). Electrokinetic confinement of axonal growth for dynamically configurable neural networks. Lab Chip 13, 589–598. doi:10.1039/c2lc41000a.
Honegger, T., Thielen, M. I., Feizi, S., Sanjana, N. E., Voldman, J., Sporns, O., et al. (2016). Microfluidic neurite guidance to study structure-function relationships in topologically-complex population-based neural networks. Sci. Rep. 6, 28384. doi:10.1038/SREP28384.
Josse, G., Seghier, M. L., Kherif, F., and Price, C. J. (2008). Explaining Function with Anatomy: Language Lateralization and Corpus Callosum Size. J. Neurosci. 28, 14132–14139. doi:10.1523/jneurosci.4383-08.2008.
Le Feber, J., Postma, W., de Weerd, E., Weusthof, M., and Rutten, W. L. C. (2015). Barbed channels enhance unidirectional connectivity between neuronal networks cultured on multi electrode arrays. Front. Neurosci. 9, 412. doi:10.3389/fnins.2015.00412.
Lodato, S., Shetty, A. S., and Arlotta, P. (2015). Cerebral cortex assembly: Generating and reprogramming projection neuron diversity. Trends Neurosci. 38, 117–125. doi:10.1016/j.tins.2014.11.003.
Pan, L., Alagapan, S., Franca, E., Brewer, G. J., and Wheeler, B. C. (2011). Propagation of action potential activity in a predefined microtunnel neural network. J. Neural Eng. 8, 046031. doi:10.1088/1741-2560/8/4/046031.
Poli, D., Wheeler, B. C., Demarse, T. B., and Brewer, G. J. (2018). Pattern separation and completion of distinct axonal inputs transmitted via micro-tunnels between co-cultured hippocampal dentate, CA3, CA1 and entorhinal cortex networks. J. Neural Eng. 15, 046009. doi:10.1088/1741-2552/aabc20.
Renault, R., Sukenik, N., Descroix, S., Malaquin, L., Viovy, J. L., Peyrin, J. M., et al. (2015). Combining microfluidics, optogenetics and calcium imaging to study neuronal communication in vitro. PLoS One 10, e0120680. doi:10.1371/journal.pone.0120680.
Roth, S., Bugnicourt, G., Bisbal, M., Gory-Fauré, S., Brocard, J., and Villard, C. (2012). Neuronal architectures with axo-dendritic polarity above silicon nanowires. Small 8, 671–675. doi:10.1002/smll.201102325.
Siddique, R., and Thakor, N. (2014). Investigation of nerve injury through microfluidic devices. J. R. Soc. Interface 11, e20130676. doi:10.1098/rsif.2013.0676.
Taylor, A. M., Dieterich, D. C., Ito, H. T., Kim, S. a., and Schuman, E. M. (2010). Microfluidic Local Perfusion Chambers for the Visualization and Manipulation of Synapses. Neuron 66, 57–68. doi:10.1016/j.neuron.2010.03.022.
Taylor, A. M., Menon, S., and Gupton, S. L. (2015). Passive microfluidic chamber for long-term imaging of axon guidance in response to soluble gradients. Lab Chip 15, 2781–2789. doi:10.1039/C5LC00503E.
Wang, L., and Marquardt, T. (2013). What axons tell each other: axon-axon signaling in nerve and circuit assembly Historical backdrop ScienceDirect. Curr. Opin. Neurobiol. 23, 974–982. doi:10.1016/j.conb.2013.08.004.
Yap, Y., and Dickson, T. (2014). Microfluidic culture platform for studying neuronal response to axonal stretch injury. Biomicrofluidics 8, 1–12, e044110. doi:10.1063/1.4891098.
Zhou, J., Wen, Y., She, L., Sui, Y. -n., Liu, L., Richards, L. J., et al. (2013). Axon position within the corpus callosum determines contralateral cortical projection. Proc. Natl. Acad. Sci. 110, E2714–E2723. doi:10.1073/pnas.1310233110.