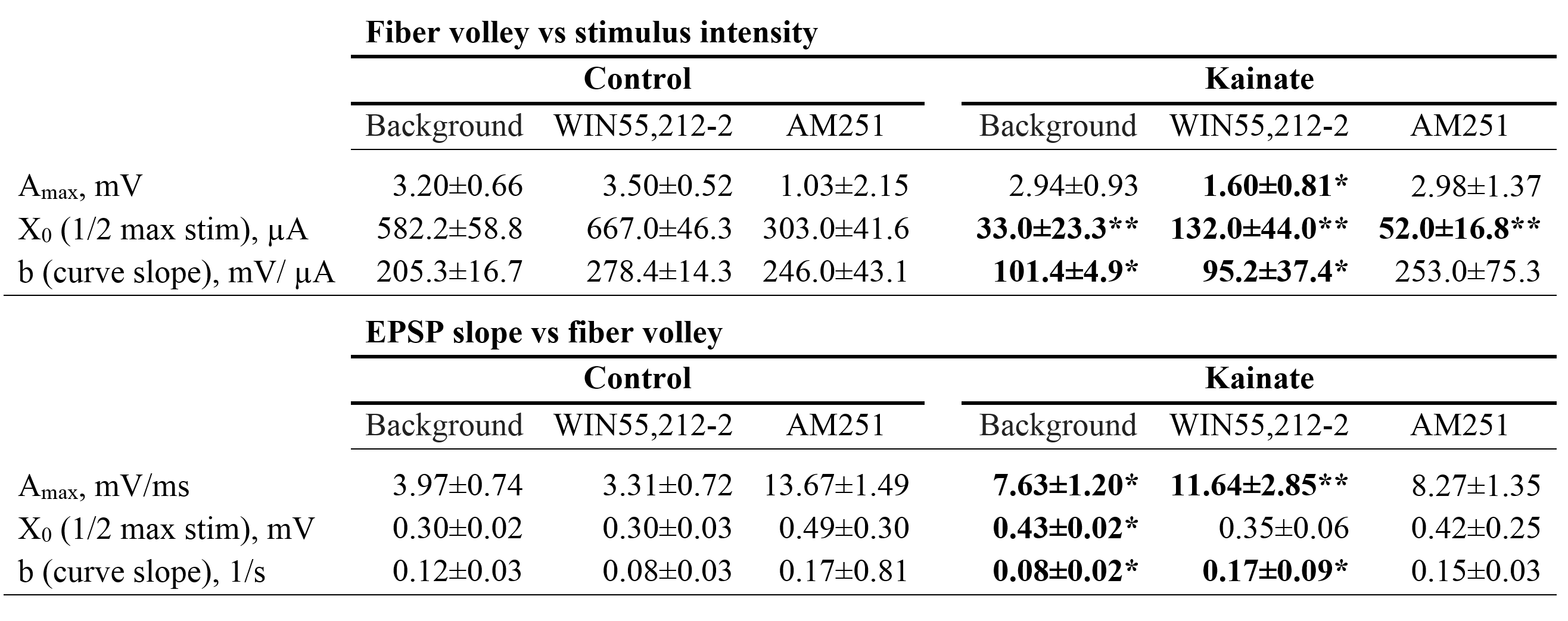
The goal of the work was to study the effect of the cannabinoid receptor agonist WIN55,212-2 and the cannabinoid type 1 receptor antagonist AM251 on electrophysiological changes in the hippocampus and the medial septal region (MS) induced by the intracerebral administration of excitotoxin kainic acid. Kainate injected into the right brain ventricle provoked persistent seizures (status epilepticus, SE) in all rats. A morphological analysis of the right hippocampus performed one month after the SE revealed the death of neurons, which was most pronounced in the hilus of the dentate gyrus and in the CA3a field of the dorsal hippocampus. In brain slices taken one month after the SE, the spontaneous activity of MS neurons and population EPSP (pEPSP) in the CA1 field of the hippocampus evoked by the stimulation of Shaffer collaterals (SC) was recorded; the changes in the activity were compared with the activity in slices of healthy animals injected with normal saline (“control slices”). It was found that the activity in MS slices from the brain of animals injected with kainic acid (“kainate slices”) was almost twice higher than in the control. After the application of WIN55,212-2, the frequency of discharges in the control did not change, whereas in kainate slices, the level of neuronal activity decreased to the control value. The application of AM251 led to an increase in the frequency of discharges in the control and its decrease in kainate slices. The registration of pEPSPs in the hippocampal slices revealed a twofold increase in the responses to SC stimulation in kainate slices compared with those in the control, i.e., an abrupt increase in neuronal excitability. A tendency for a decrease in excitability after the application of WIN55,212-2 and, conversely, for its increase by the action of AM251 was noted in evoked responses in the hippocampal kainate slices. Our results allow to assume the protective impact of cannabinoid agonist WIN55,212-2 on neuronal activity in the medial septum and hippocampus that disturbed by neurotoxic kainate influence.

| Attachment | Size |
|---|---|
| 1.16 MB |
This study was supported by the Russian Foundation for Basic Research (project no. 18-015-00157) and Regional Grant “r-a” (project no. 17-44-500312). The authors are grateful to S.V. Sidorova for reading the manuscript.
Alger B.E. 2002. Retrograde signaling in the regulation of synaptic transmission: focus on endocannabinoids. Prog. Neurobiol. 68, 247-286.
Ameri A., Simmet T. 2000. Effects of 2-arachidonylglycerol, an endogenous cannabinoid, on neuronal activity in rat hippocampal slices. Arch. Pharmacol. 361, 265-272.
Ameri A., Wilhelm A., Simmet T. 1999. Effects of the endogenous cannabinoid, anandamide, on neuronal activity in rat hippocampal slices. Br. J. Pharmacol. 126, 1831–1839.
Astasheva E., Astashev M., Kitchigina V. 2015. Changes in the behavior and oscillatory activity in cortical and subcortical brain structures induced by repeated L-glutamate injections to the medial septal area in guinea pigs. Epilepsy Res., 109, 134-145.
Ben-Ari Y., Tremblay E., Ottersen O.P. 1980. Injections of kainic acid into the amygdaloid complex of the rat: An electrographic, clinical and histological study in relation to the pathology of epilepsy. Neuroscience 5, 515–528.
Berrendero F., Romero J., García-Gil L., Suarez I., De la Cruz P., Ramos J.A, Fernández-Ruiz J.J. 1998. Changes in cannabinoid receptor binding and mRNA levels in several brain regions of aged rats. Biochim Biophys Acta 1407, 205-214.
Bisogno T., Berrendero F., Ambrosino G., Cebeira M., Ramos J.A., Fernandez-Ruiz J.J., Di Marzo V. 1999. Brain regional distribution of endocannabinoids: implications for their biosynthesis and biological function. Biochem. Biophys. Res Commun. 256, 377-380.
Bobrov M.Y., Lizhin A.A., Andrianova E.L., Gretskaya N.M., Frumkina L.E., Khaspekov L.G., Bezuglov V.V. 2008. Antioxidant and neuroprotective properties of N-arachidonoyldopamine. Neurosci. Lett. 43, 6-11.
Braakman H.M., van Oostenbrugge R.J., van Kranen-Mastenbroek V.H., de Krom M.C. 2009. Rimonabant induces partial seizures in a patient with a history of generalized epilepsy. Epilepsia 50, 2167–2173.
Bragin A., Engel J. Jr., Wilson C.L., Vizentin E., Mathern G.W. 1999. Electrophysiologic analysis of a chronic seizure model after unilateral hippocampal KA injection. Epilepsia 40, 1210-1221.
Brashear H.R., Zaborszky L., Heimer L. 1986. Distribution of GABAergic and cholinergic neurons in the rat diagonal band. Neuroscience 17, 439-451.
Brauer K., Seeger G., Hartig W., Rossner S., Poethke R., Kacza J., Schliebs R., Bruckner G., Bigl V. 1998. Electron microscopic evidence for a cholinergicinnervation of GABAergic parvalbumin-immunoreactive neurons in the rat medial septum. J. Neurosci. Res., 54, 248–253.
Buonamici M., Young G.A., Khazan N. 1982. Effects of acute delta 9-THC administration on EEG and EEG power spectra in the rat. Neuropharmacology 21, 825-829.
Butuzova M.V., Kitchigina V.F. 2008. Repeated blockade of GABAA receptors in the medial septal region induces the epileptiform activity in the hippocampus. Neurosci. Lett. 434, 133-138.
Colom LV, García-Hernández A, Castañeda MT, Perez-Cordova MG, Garrido-Sanabria ER. 2006. Septo-hippocampal networks in chronically epileptic rats: Potential antiepileptic effects of theta rhythm generation. J. Neurophysiol., 95, 3645–3653.
Coomber B., O’Donoghue F.M., Mason R. 2008. Inhibition of endocannabinoid metabolism attenuates enhanced hippocampal neuronal activity induced by kainic acid. Synapse 62, 746–755.
Cossart, R., Tyzio, R., Dinocourt, C., Esclapez, M., Hirsch, J.C., Ben-Ari, Y., Bernard, C., 2001. Dendritic but not somatic GABAergic inhibition is decreased in experimental epilepsy. Nat. Neurosci. 4, 52–62.
Di Marzo V., Melck D., Bisogno T., De Petrocellis L. 1998. Endocannabinoids: endogenous cannabinoid receptor ligands with neuromodulatory action. Trends. Neurosci. 21:521–528.
Egertová M., Elphick M.R. 2000. Localisation of cannabinoid receptors in the rat brain using antibodies to the intracellular C-terminal tail of CB1. J. Comp. Neurol. 422, 159-171.
Esch T., Michalsen A., Stefano G.B. 2006. Endocannabinoids as molecular instruments of health promotion. Med. Monatsschr. Pharm. 29, 397-403.
Falenski K.W., Blair R.E., Sim-Selley L.J., Martin B.R., DeLorenzo R.J. 2007. Status epilepticus causes a long-lasting redistribution of hippocampal cannabinoid type 1 receptor expression and function in the rat pilocarpine model of acquired epilepsy. Neuroscience 146, 1232–1244.
Falenski K.W., Carter D.S., Harrison A.J., Martin B.R., Blair R.E., DeLorenzo R.J. 2009. Temporal characterization of changes in hippocampal cannabinoid CB1(1) receptor expression following pilocarpine-induced status epilepticus. Brain Res. 1262, 64–72.
Felder C.C., Glass M. 1998. Cannabinoid receptors and their endogenous agonists. Annu Rev. Pharmacol. Toxicol. 38:179–200.
Fleck M., Hirotsune S., Gambello M., Phillips-Tansey E., Suares G., Mervis R., Wynshaw-Boris A., McBain C. 2000. Hippocampal abnormalities and enhanced excitability in a murine model of human lissencephaly. J. Neurosci. 20 (7): 2439-2450.
Follesa, P., Tarantino, A., Floris, S., Mallei, A., Porta, S., Tuligi, G., Cagetti, E., Caddeo, M., Mura, A., Serra, M., Biggio, G., 1999. Changes in the gene expression of GABAA receptor subunit mRNAs in the septum of rats subjected to pentylenetetrazolinduced kindling. Brain Res. Mol. Brain Res. 70, 1-8.
Freund T.F., Katona I., Piomelli D. 2003. Role of endogenous cannabinoids in synaptic signaling. Physiol. Rev. 83, 1017–1066.
Gao B., Hornung J.P., Frischy J.M. 1995. Identification of distinct GABAA-receptor subtypes in cholinergic and parvalbumin-positive neurons of the rat and marmosed medial septum-diagonal band complex. Neuroscience 65, 101-117.
Garrido-Sanabria E.R., Castaneda M.T., Banuelos C., Perez-Cordova M.G., Hernandez S., Colom L.V. 2006. Septal GABAergic neurons are selectively vulnerable to pilocarpine induced status epilepticus and chronic spontaneous seizures. Neuroscience 142, 871–883.
Gonzalez S., Fernandez-Ruiz J., Sparpaglione V., Parolaro D., Ramos J.A. 2002. Chronic exposure to morphine, cocaine or ethanol in rats produced different effects in brain cannabinoid CB1(1) receptor binding and mRNA levels. Drug Alcohol Depend. 66, 77-84.
Goonawardena A.V., Riedel G., Hampson R.E. 2011. Cannabinoids alter spontaneous firing, bursting, and cell synchrony of hippocampal principal cells. Hippocampus 21, 520-531.
Gordon RY, Shubina LV, Kapralova MV, Pershina EB, Khutzian SS, Arhipov VI. 2014. Peculiarities of neurodegeneration in hippocampus fields after kainic acid action in rats. Tsitologiia 56, 919-925 (Russian).
Gritti I., Mainville L., Jones B.E. 1993. Codistribution of GABA- with acetylcholine-synthesizing neurons in the basal forebrain of the rat. J. Comp. Neurol. 329, 438-457.
Hajzsan T., Alreja M., Leranth C. 2004. Intrinsic vesicular glutamate transporter 2-immunoreactive input to septohippocampal parvalbumin-containing neurons: novel glutamatergic local circuit cells. Hippocampus 14, 499-509.
Hansen H.H., Schmid P.C., Bittigau P., Lastres-Becker I., Berrendero F., Manzanares J., Ikonomidou C., Schmid H.H., Fernández-Ruiz J.J., Hansen H.S. 2001. Anandamide, but not 2 arachidonoylglycerol, accumulates during in vivo neurodegeneration. J. Neurochem. 78, 1415–1427.
Hart C.L., Ilan A.B., Gevins A., Gunderson E.W., Role K., Colley J., Foltin R.W. 2010. Neurophysiological and cognitive effects of smoked marijuana in frequent users. Pharmacol. Biochem. Behav. 96, 333-341.
Hauser W.A., Hersdorffer D.C. 1990. Epilepsy: Frequency, Causes, and Consequences. Demos, New York. 1990.
Heinemann U., Beck H., Dreier J.P., Ficker E., Stabel J., Zhang C.L. 1992. The dentate gyrus as a regulated gate for the propagation of epileptiform activity. Epilepsy Res. Suppl. 7, 273-280.
Henderson Z., Fiddler G., Saha S., Boros A., Halasy K. 2004. A parvalbumin-contaning, axosomatic synaptic network in the rat medial septum: relevance to rhythmogenesis. European J. Neurosci. 19, 2753-2768.
Henderson Z., Morris N.P., Grimwood P., Fiddler G., Yang H.W. 2001. Appenteng K. Morfology of local axon collaterals of electrophysiologically characterised neurons in the rat medial septum/diagonal band complex. J. Comp. Neurol. 430, 410-432.
Herkenham M., Lynn A.B., Johnson M.R., Melvin L.S., de Costa B.R., Rice K.C. 1991. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J. Neurosci. 11, 563-583.
Hofmann M.E., Nahir B., Frazier C.J. 2008. Excitatory afferents to CA3 pyramidal cells display differential sensitivity to CB1 dependent inhibition of synaptic transmission. Neuropharmacology 55, 1140-1146.
Hrabovszky E., Wittmann G., Kalló I., Füzesi T., Fekete C., Liposits Z. 2012. Distribution of type 1 cannabinoid receptor-expressing neurons in the septal-hypothalamic region of the mouse: colocalization with GABAergic and glutamatergic markers. J. Comp. Neurol. 520, 1005-1020.
Hsu D. 2007. The dentate gyrus as a filter or gate: a look back anda look ahead. Prog. Brain Res. 163, 601–613.
Ilan A.B., Smith M.E., Gevins A. 2004. Effects of marijuana on neurophysiological signals of working and episodic memory. Psychopharmacology (Berl). 176, 214-222.
Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M, Watanabe M. 2009. Endocannabinoid-mediated control of synaptic transmission. Physiol. Rev. 89, 309-80.
Karanian D.A., Brown Q.B., Makriyannis A., Kosten T.A., Bahr B.A. Dual modulation of endocannabinoid transport and fatty-acid amide hydrolase protects against excitotoxicity. J. Neurosci. 2005. 25, 7813–7820.
Karlócai M.R., Tóth K., Watanabe M., Ledent C., Juhász G., Freund T.F., Maglóczky Z. 2011.Redistribution of CB1 cannabinoid receptors in the acute and chronic phases of pilocarpine-induced epilepsy. PLoS One. 6(11):e27196.
Karr L., Pan Y.Z., Rutecki P.A. 2010. CB1 receptor antagonism impairs the induction of epileptiform activity by group I metabotropic glutamate receptor activation. Epilepsia. 51 (S3), 121-125.
Katona I., Freund T.F. 2008. Endocannabinoid signaling as a synaptic circuit breaker in neurological disease. Nat. Med. 14, 923-930.
Kawamura Yю, Fukaya Mю, Maejima Tю, Yoshida Tю, Miura Eю, Watanabe Mю, Ohno-Shosaku Tю, Kano M. 2006. The CB1 cannabinoid receptor is the major cannabinoid receptor at excitatory presynaptic sites in the hippocampus and cerebellum. J. Neurosci. 26, 2991–3001.
Khaspekov L.G., BrenzVerca M.S. , Frumkina L.E., Hermann H., Marsicano G., Lutz B. 2004. Involvement of brain-derived neurotrophic factor in cannabinoid receptor-dependent protection against excitotoxicity. Eur. J. Neurosci. 19, 1691–1698.
Kiss, J., Patel, A.J., Freund, T.F., 1990. Distribution of septohippocampal neurons containing parvalbumin or cholinacetytransferase in the rat brain. J. Comp. Neurol. 298, 362–372.
Kitchigina V.F., Butuzova M.V. 2009. Theta activity of septal neurons during different epileptic phases: The same frequency but different significance? Exp. Neurol. 216, 449-458.
Krook-Magnuson E, Armstrong C, Bui A, Lew S, Oijala M, Soltesz I. 2015. In vivo evaluation of the dentate gate theory in epilepsy. J. Physiol. 593, 2379-88.
Leranth C., Frotscher M. 1989. Organization of the septal region in the rat brain: chilinergic-GABAergic interconnections and the termination of hippocampo-septal fibers. J. Comp. Neurol. 289, 304-314.
Maglоczky Z., Tóth K., Karlócai R., Nagy S., Eross L., Czirják S., Vajda J., Rásonyi G., Kelemen A., Juhos V., Halász P., Mackie K., Freund T.F. 2010. Dynamic changes of CB1-receptor expression in hippocampi of epileptic mice and humans. Epilepsia 51(S3), 115-120.
Maier N., Morris G., Schuchmann S., Korotkova T. 2012. Ponomarenko A., Böhm C., Wozny C., Schmitz D. Cannabinoids disrupt hippocampal sharp wave-ripples via inhibition of glutamate release. Hippocampus 22, 1350–1362.
Mailleux P., Vanderhaeghen J.J. 1992. Distribution of neuronal cannabinoid receptor in the adult rat brain: a comparative receptor binding radioautography and in situ hybridization histochemistry. Neuroscience 48, 655-68.
Malkov A.E., Popova I.Yu. 2011. Functional changes in the septal GABAergic system of animals with a model of temporal lobe epilepsy. Gen. Physiol. Biophys. 30, 310–320.
Mal'kov A.E., Karavaev E.N., Popova I.Y., Kichigina V.F. 2008. Changes in oscillatory activity of neurons in the medial septal area in animals with a model of chronic temporal epilepsy. Neurosci. Behav. Physiol. 38, 995-959.
Maroof N., Pardon M.C., Kendall D.A. 2013. Endocannabinoid signalling in Alzheimer's disease. Biochem. Soc. Trans. 41, 1583-1587.
Marsicano G, Lutz B. 1999. Expression of the cannabinoid receptor CB1 in distinct neuronal subpopulations in the adult mouse forebrain. Eur. J. Neurosci. 11, 4213-425.
Marsicano G., Goodenough S., Monory K., Hermann H., Eder M., Cannich A., Azad S.C., Cascio M.G., Gutierrez S.O., van der Stelt M., Lopez-Rodriguez M.L., Casanova E., Schutz G., Zieglgansberger W., Di Marzo V., Behl C., Lutz B. 2003. CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science 302, 84–88.
Matsuda L.A., Bonner T.I., Lolait S.J. 1993. Localization of cannabinoid receptor mRNA in rat brain. J Comp Neurol. 327, 535-550.
Matsuda L.A., Lolait S.J., Brownstein M.J., Young A.C., Bonner T.I. 1990. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 346, 561–564.
McNamara J.O. 1994. Cellular and molecular basis of epilepsy. J. Neurosci. 14, 3413-3425.
Miller J.W., Turner G.M., Gray B.C. 1994. Anticonvulsant effects of the experimental induction of hippocampal theta activity. Epilepsy Res. 18, 195-204.
Misner D.L., Sullivan J.M. 1999. Mechanism of cannabinoid effects on long-term potentiation and depression in hippocampal CA1 neurons. J. Neurosci. 19, 6795-6805.
Monory K., Massa F., Egertova M., Eder M., Blaudzun H., Westenbroek R., Kelsch W., Jacob W., Marsch R., Ekker M., Long J., Rubenstein J.L., Goebbels S., Nave K.A., During M., Klugmann M., Wölfel B., Dodt H.U., Zieglgänsberger W., Wotjak C.T., Mackie K., Elphick M.R., Marsicano G., Lutz B. 2006. The endocannabinoid system controls key epileptogenic circuits in the hippocampus. Neuron 51, 455–466.
Morimoto K., Fahnestock M., Racine R.J. 2004. Kindling and status epilepticus models of epilepsy: rewiring the brain. Prog. Neurobiol. 73, 1-60.
Mufson E.J ., Ginsberg S.D., Ikonomovic M.D., DeKosky S.T. 2003. Human cholinergic basal forebrain: chemoanatomy and neurologic dysfunction. J Chem Neuroanat. 26, 233-242.
Munro S., Thomas K.L., Abu-Shaar M. 1993. Molecular characterization of a peripheral receptor for cannabinoids. Nature 365, 61–65.
Norris C., Scheff W. 2009. Recovery of afferent function and synaptic strength in hippocampal CA1 following traumatic brain injury. Neurotrauma 26, 2269-2278.
Nyiri G., Szabadits E., Cserep C., Mackie K., Shigemoto R., Freund T.F. 2005. GABAB and CB1 cannabinoid receptor expression identifies two types of septal cholinergic neurons. Eur. J. Neurosci. 21, 3034 –3042.
Ohno-Shosaku T., Tsubokawa H., Mizushima I., Yoneda N., Zimmer A., Kano M. 2002. Presynaptic cannabinoid sensitivity is a major determinant of depolarization-induced retrograde suppression at hippocampal synapses. J. Neurosci. 22, 3864-3872.
Paton G.S., Pertwee R.G., Davies S.N. 1998. Correlation between cannabinoid mediated effects on paired pulse depression and induction of long term potentiation in the rat hippocampal slice. Neuropharmacology 37:1123–1130.
Paxinos G., Watson C. 1998. The rat brain in stereotaxic coordinates. Sydney: Academic press. 321 p.
Popova I.Y., Sinelnikova V.V., Kitchigina V.F. 2008. Disturbance of the correlation between hippocampal and septal EEGs during epileptogenesis. Neurosci. Lett. 442, 228–233.
Raza M, Pal S, Rafiq A, DeLorenzo RJ. 2001. Long-term alteration of calcium homeostatic mechanisms in the pilocarpine model of temporal lobe epilepsy. Brain Res. 903, 1-12.
Robbe D, Montgomery SM, Thome A, Rueda-Orozco PE, McNaughton BL, Buzsaki G. 2006 Cannabinoids reveal importance of spike timing coordination in hippocampal function. Nat. Neurosci. 9, 1526-1533.
Robbe D., Buzsáki G. 2009. Alteration of theta timescale dynamics of hippocampal place cells by a cannabinoid is associated with memory impairment. J. Neurosci. 29, 12597-12605.
Romero J., Garcia-Palomero E., Castro J.G., Garcia-Gil L., Ramos J.A., Fernandez-Ruiz J.J. 1997. Effects of chronic exposure to delta9-tetrahydrocannabinol on cannabinoid receptor binding and mRNA levels in several rat brain regions. Brain Res. Mol. Brain Res. 46, 100-108.
Schmued L.C., Hopkins K.J. 2000. Fluoro-Jade B: a high affinity fluorescent marker for the localization of neuronal degeneration. Brain Res. 874, 123-30.
Shen M., Piser T.M., Seybold V.S., Thayer S.A. 1996. Cannabinoid receptor agonists inhibit glutamatergic synaptic transmission in rat hippocampal cultures. J. Neurosci. 16, 4322–4334.
Shubina L., Aliev R., Kitchigina V. 2015. Attenuation of kainic acid-induced status epilepticus by inhibition of endocannabinoid transport and degradation in guinea pigs. Epilepsy Res. 111, 33-44.
Sloviter R.S., 1991. Permanently altered hippocampal structure, excitability, and inhibition after experimental status epilepticus in the rat: the "dormant basket cell" hypothesis and its possible relevance to temporal lobe epilepsy. Hippocampus 1, 41-66.
Sloviter R.S., Zappone C.A., Harvey B.D., Bumanglag A.V., Bender R.A., Frotscher M. 2003. "Dormant basket cell" hypothesis revisited: relative vulnerabilities of dentate gyrus mossy cells and inhibitory interneurons after hippocampal status epilepticus in the rat. J. Comp. Neurol.459, 44-76.
Soltesz I., Alger B.E., Kano M., Lee S.H., Lovinger D.M., Ohno-Shosaku T., Watanabe M. 2015. Weeding out bad waves: towards selective cannabinoid circuit control in epilepsy. Nat. Rev. Neurosci. 16, 264-277.
Sotty F., Danik M., Manseau F., Laplante F., Quirion R., Williams S. 2003. Glutamatergic, cholinergic and GABAergic neurons contribute to the septohippocampal pathway and exhibit distinct electrophysiological properties: novel implications for hippocampal rhythmicity. J. Physiol. 551, 927-943.
Stella N., Schweitzer P., Piomelli D. 1997. A second endogenous cannabinoid that modulates long-term potentiation. Nature 388(6644), 773-778.
Sullivan J .M. 1999. Mechanisms of cannabinoid-receptor-mediated inhibition of synaptic transmission in cultured hippocampal pyramidal neurons. J. Neurophysiol. 82, 1286–1294.
Tsou K., Brown S., Sañudo-Peña M.C., Mackie K., Walker J.M. 1998. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 83, 393-411.
van Rijn, C.M., Perescis, M.F., Vinogradova, L., van Luijtelaar, G. 2011. Endocannabinoid system protects against cryptogenic seizures. Pharmacol. Rep. 63, 165-168.
Vinogradova L.V., van Rijn C.M. 2015. Long-term disease-modifying effect of the endocannabinoid agonist WIN55,212-2 in a rat model of audiogenic epilepsy. Pharmacol. Rep. 67, 501-503.
Wilson R.I., Nicoll R.A. 2002. Endocannabinoid signaling in the brain. Science. 296, 678 –682.
Wolf O.T., Dyakin V., Patel A., Vadasz C., de Leon M.J., McEwen B.S., Bulloch K. 2002. Volumetric structural magnetic resonance imaging (MRI) of the rat hippocampus following kainic acid (KA) treatment. Brain Res. 934, 87-96.
Wu K., Leung L.S. 2003. Increased dendritic excitability in hippocampal ca1 in vivo in the kainic acid model of temporal lobe epilepsy: a study using current source density analysis. Neuroscience 116, 599-616.
Wu M., Hajzsan T., Leranth C., Alreja M. 2003. Nicotine recruits a local glutamatergic circuit to excite septohippocampal GABAergic neurons. European J. Neurosci. 18, 1155-1168.
Zaborszky L., Pang K., Somogyi J., Nadasdy Z., Kallo I. 1999. The basal forebrain corticopetal system revisited. Ann. N Y Acad. Sci. 87, 339-367.