Neurons of the substantia nigra are most prone to degeneration in Parkinson’s disease. The cause of their vulnerability remains unclear and knowledge of the molecular and microstructural features of the substantia nigra pars compacta will help understanding why nigral neurons are vulnerable to damaging factors.
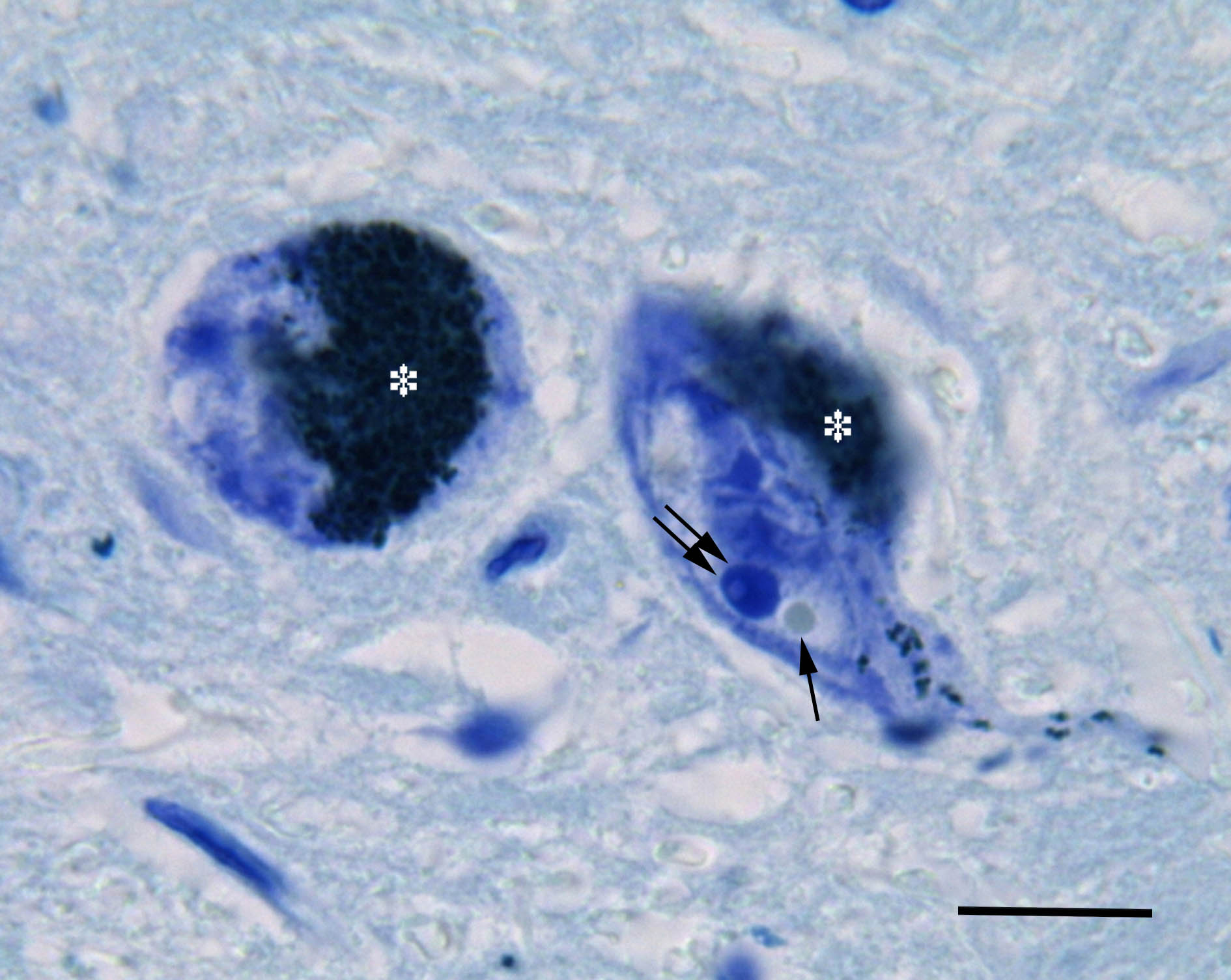
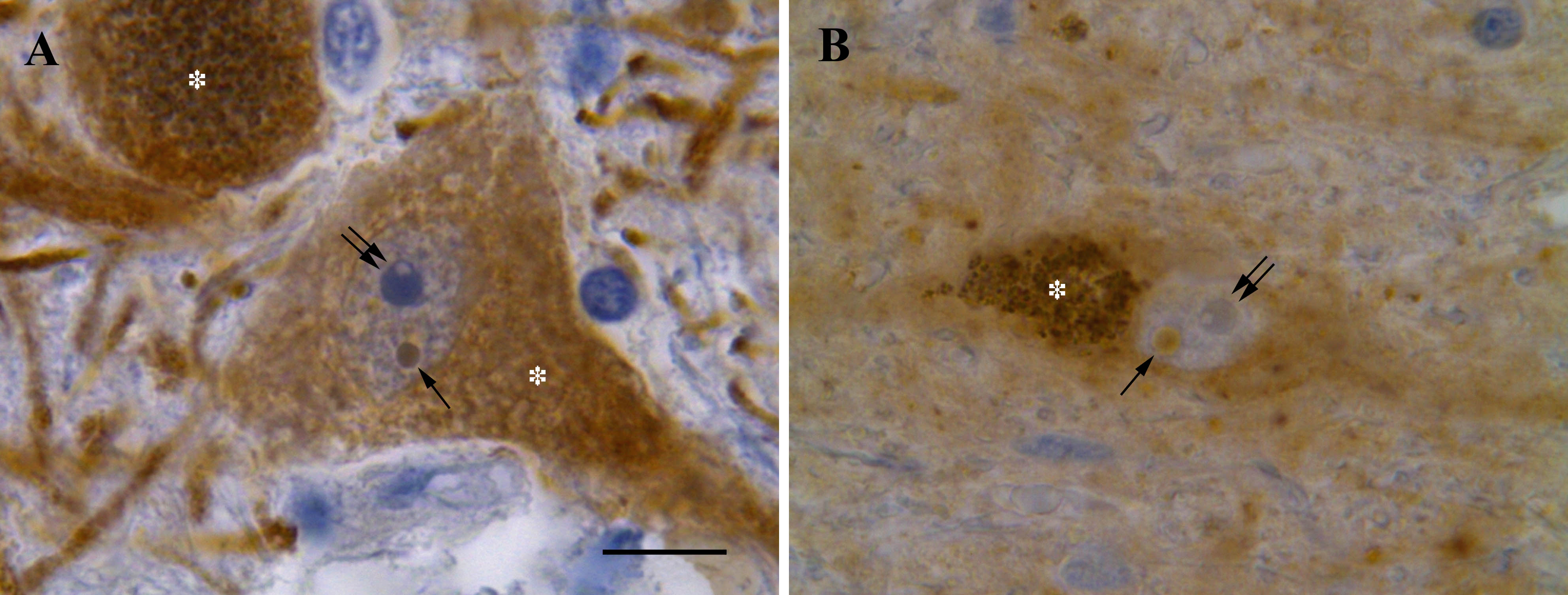
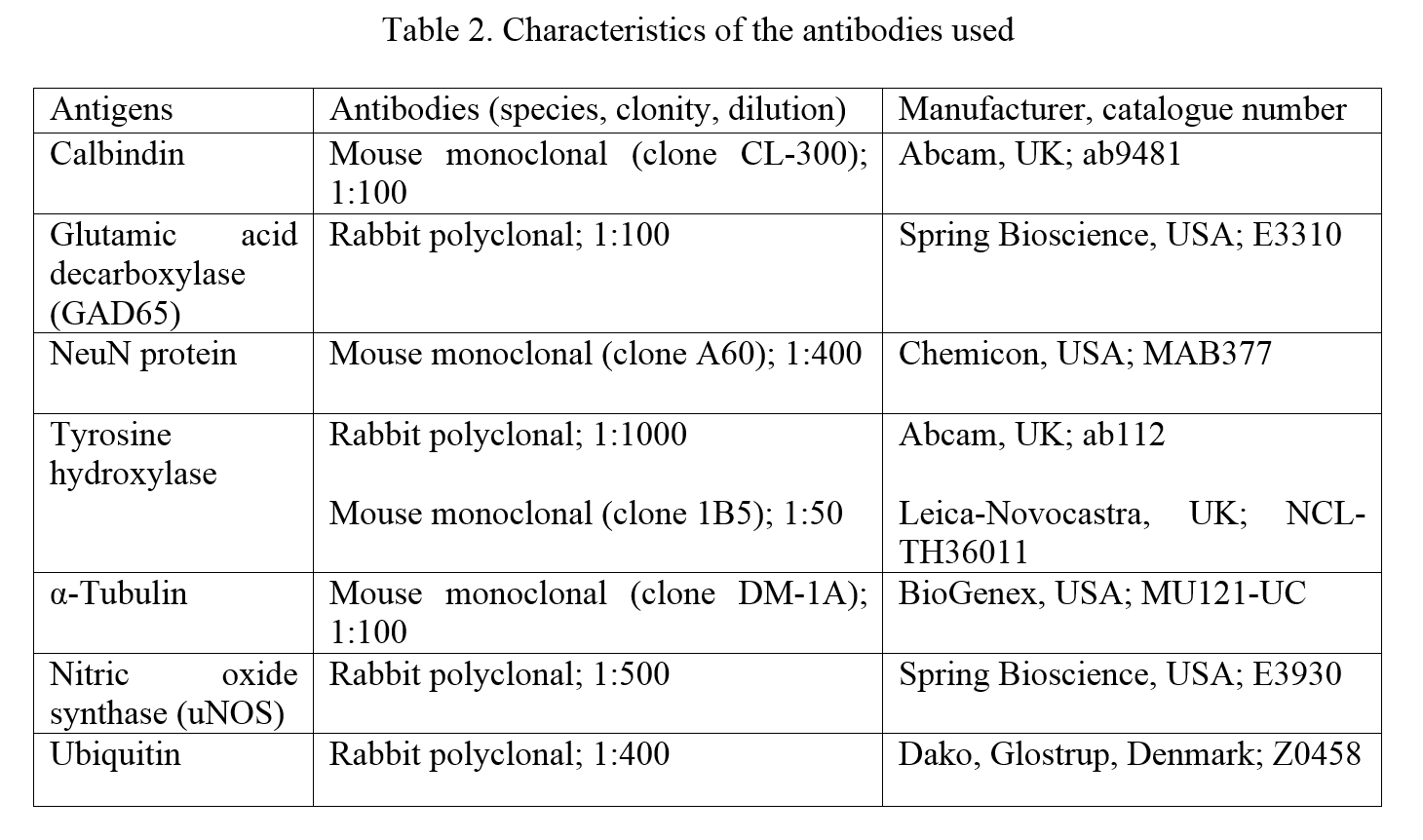
The present study was aimed to investigate the intranuclear inclusions of the nigral neurons, the Marinesco bodies and the Roncoroni rodlets, which origin and function are uncertain, using ubiquitin-, tyrosine hydroxylase-, nitric oxide synthase-, calbindin-, NeuN-, glutamic acid decarboxylase-, and α-tubulin-immunohistochemistry and iron histochemistry with DAB enhancement.
Of the tested substances, tyrosine hydroxylase and nitric oxide synthase were revealed for the first time in the Marinesco bodies. Non-heme iron was found for the first time in both the Marinesco bodies and the Roncoroni rodlets. In accordance with previous studies, ubiquitin-immunoreactivity was demonstrated in the Marinesco bodies. Moreover, we describe some smaller round and dot-like ubiquitin-immunoreactive structures in the nucleus of melanized neurons. The found small ubiquitin-immunopositive structures within the nucleus are proposed to be the developmental stages of growing Marinesco bodies, whereas Marinesco bodies themselves seem to label the neurons with impaired function of proteasome.
| Attachment | Size |
|---|---|
| 6.15 MB |
ALLADI P.A., MAHADEVAN A., VIJAYALAKSHMI K., MUTHANE U., SHANKAR S.K. & RAJU T.R. (2010): Ageing enhances alpha-synuclein, ubiquitin and endoplasmic reticular stress protein expression in the nigral neurons of Asian Indians. Neurochemistry International 57, 530-539.
ANDERSEN J.K. (2004): Iron dysregulation and Parkinson's disease. Journal of Alzheimer's Disease 6, S47-52.
ANRAKU S., MIURA C., MORI H. & FUKUTOMI T. (1970): Histological studies on the intranuclear inclusions of the melanin-pigmented nerve cells in the substantia nigra and the nucleus loci caerulei. Kurume Medical Journal 17, 211-224.
BEARD J. L., CONNOR J. R. & JONES B. C. (1993): Brain iron: location and function. Progress in Food & Nutrition Science 17, 183–221.
BENKOVIC S.A. & CONNOR J.R. (1993): Ferritin, transferrin, and iron in selected regions of the adult and aged rat brain. Journal of Comparative Neurology 338, 97-113.
BENARROCH E.E. (2009): Brain iron homeostasis and neurodegenerative disease. Neurology 72, 1436-1440.
BEN-SHACHAR D., RIEDERER P. & YOUDIM M.B. (1991): Iron-melanin interaction and lipid peroxidation: implications for Parkinson's disease. Journal of Neurochemistry 57, 1609-1614.
BERG D., RIEDERER P. & GERLACH M. (2008): Contribution of disturbed iron metabolism to the pathogenesis of Parkinson’s disease. Future Neurology 3, 447-461.
CAVALCANTI-KWIATKOSKI R., RAISMAN-VOZARI R., GINESTET L. & DEL BEL E. (2010): Altered expression of neuronal nitric oxide synthase in weaver mutant mice. Brain Research 1326, 40-50.
CHEEPSUNTHORN P., PALMER C. & CONNOR J.R. (1998): Cellular distribution of ferritin subunits in postnatal rat brain. Journal of Comparative Neurology 400, 73–86.
CONNOR J.R., MENZIES S.L., BURDO J.R. & BOYER P.J. (2001): Iron and iron management proteins in neurobiology. Pediatric Neurology 2001; 25, 118-129.
CZARNECKA A., LENDA T., DOMIN H., KONIECZNY J., SMIALOWSKA M. & LORENC-KOCI E. (2013): Alterations in the expression of nNOS in the substantia nigra and subthalamic nucleus of 6-OHDA-lesioned rats: the effects of chronic treatment with l-DOPA and the nitric oxide donor, molsidomine. Brain Research 1541, 92-105.
DICKSON D.W., WERTKIN A., KRESS Y., KSIEZAK-REDING H. & YEN S.H. (1990): Ubiquitin immunoreactive structures in normal human brains. Distribution and developmental aspects. Laboratory Investigation 63, 87-99.
D’ISCHIA M. & PROTA G. (1997): Biosynthesis, structure, and function of neuromelanin and its relation to Parkinson’s disease: A Critical Update. Pigment Cell Research 10, 470-476.
DOUBLE K.L., GERLACH M., SCHÜNEMANN V., TRAUTWEIN A.X., ZECCA L., GALLORINI M., YOUDIM M.B., RIEDERER P. & BEN-SHACHAR D. (2003): Iron-binding characteristics of neuromelanin of the human substantia nigra. Biochemical Pharmacology 66, 489-494.
DWORK A.J., SCHON E.A. & HERBERT.J. (1988): Nonidentical distribution of transferrin and ferric iron in human brain. Neuroscience 27, 333-345.
ENGELEN M., VANNA R., BELLEI C., ZUCCA F.A., WAKAMATSU K., MONZANI E., ITO S., CASELLA L. & ZECCA L. (2012): Neuromelanins of human brain have soluble and insoluble components with dolichols attached to the melanic structure. Public Library of Science One 7, e48490.
FAUCHEUX B.A., MARTIN M.E., BEAUMONT C., HAUW J.J., AGID Y. & HIRSCH E.C. (2003): Neuromelanin associated redox-active iron is increased in the substantia nigra of patients with Parkinson's disease. Journal of Neurochemistry 86, 1142-1148.
GEUENS E., BROUNS I., FLAMEZ D., DEWILDE S., TIMMERMANS J.P. & MOENS L. (2003): A globin in the nucleus! Journal of Biological Chemistry 278, 30417-30420.
GLINKA Y., GASSEN M. & YOUDIM M.B.H. (1997): Iron and neurotransmitter function in the brain. In: Metals and Oxidative Damage in Neurological Disorders (Ed Connor J.R.), pp. 1-22, New York: Springer Science Media.
GONZÁLEZ-HERNÁNDEZ T., ABDALA P. & RODRÍGUEZ M. (1997): NOS expression in nigral cells after excitotoxic and non-excitotoxic lesion of the pedunculopontine tegmental nucleus. European Journal of Neuroscience 9, 2658-2667.
GÖTZ M.E., DOUBLE K., GERLACH M., YOUDIM M.B. & RIEDERER P. (2004): The relevance of iron in the pathogenesis of Parkinson's disease. Annals of the New York Academy of Sciences 1012, 193-208.
GRIGORIEV I.P., SUKHORUKOVA E.G., KOLOS E.A. & KORZHEVSKII D.E. (2013): Neuromelanin in substantia nigra neurons lacking tyrosine hydroxylase. Neuroscience and Behavioral Physiology 43, 461-463.
GRIGORIEV I.P., VASILENKO M.S., SUKHORUKOVA E.G. & KORZHEVSKII D.E. (2012): Use of different antibodies to tyrosine hydroxylase to study catecholaminergic systems in the mammalian brain. Neuroscience and Behavioral Physiology 42, 210-213.
GRIZZI F., CEVA-GRIMALDI G., FRANCESCHINI B., RONCALLI M., CHIRIVA-INTERNATI M. & DIOGUARDI N. (2002): Simultaneous staining of cytoplasmic iron and collagen matrix in human liver biopsy specimens. European Journal of Histochemistry 46, 101-104.
HERSHKO A. & CIECHANOVER A. (1998): The ubiquitin system. Annual Review of Biochemistry 67, 425–79.
JELLINGER K., KIENZL E., RUMPELMAIR G., RIEDERER P., STACHELBERGER H., BEN-SHACHAR D. & YOUDIM M.B. (1992): Iron-melanin complex in substantia nigra of parkinsonian brains: an x-ray microanalysis. Journal of Neurochemistry; 59, 1168-1171.
JIN L., WANG J., JIN H., FEI G., ZHANG Y., CHEN W., ZHAO L., ZHAO N., SUN X., ZENG M. & ZHONG C. (2012): Nigral iron deposition occurs across motor phenotypes of Parkinson's disease. European Journal of Neurology 19, 969-976.
KAUR D. & ANDERSEN J. (2004): Does cellular iron dysregulation play a causative role in Parkinson's disease? Ageing Research Reviews 3, 327-343.
KE Y. & MING QIAN Z. (2003): Iron misregulation in the brain: a primary cause of neurodegenerative disorders. The Lancet Neurology 2, 246-253.
KONDO Y., OGAWA N., ASANUMA M., OTA Z. & MORI A. (1995): Regional differences in late-onset iron deposition, ferritin, transferrin, astrocyte proliferation, and microglial activation after transient forebrain ischemia in rat brain. Journal of Cerebral Blood Flow & Metabolism 15, 216-226.
KORZHEVSKII D.E., GRIGOR’EV I.P., KIRIK O.V. & ALEKSEEVA O.S. (2015): Neuroglobin distribution in the rat cerebellar Purkinje cells. Journal of Evolutionary Biochemistry and Physiology 51, 517-519.
KORZHEVSKII D.E., GRIGOR'EV I.P., SUKHORUKOVA E.G. & GUSEL'NIKOVA V.V. (2017): [Immunohistochemical characteristics of the substantia nigra neurons of the human]. Russian. Zhurnal Nevrologii i Psikhiatrii imeni S.S. Korsakova 117, 50-55.
KORZHEVSKII D.E., SUKHORUKOVA E.G., GILEROVICH E.G., PETROVA E.S., KIRIK O.V. & GRIGOREV I.P. (2014): Advantages and disadvantages of zinc-ethanol-formaldehyde as a fixative for immunocytochemical studies and confocal laser microscopy. Neuroscience Behavioral Physiology 44, 542-545.
KORZHEVSKII D.E., SUKHORUKOVA E.G., KIRIK O.V. & GRIGOREV I.P. (2015): Immunohistochemical demonstration of specific antigens in the human brain fixed in zinc-ethanol-formaldehyde. European Journal of Histochemistry 59, 2530.
LAN J. & JIANG D.H. (1997): Excessive iron accumulation in the brain: a possible potential risk of neurodegeneration in Parkinson’s disease. Journal of Neural Transmission 104, 649–660.
LEESTMA J.E. & ANDREWS J.M. (1969): The fine structure of the Marinesco body. Archives of Pathology 88, 431-436.
LI W., GARRINGER H.J., GOODWIN C.B., RICHINE B., ACTON A., VANDUYN N., MUHOBERAC B.B., IRIMIA-DOMINGUEZ J., CHAN R.J., PEACOCK M., NASS R., GHETTI B. & VIDAL R. (2015): Systemic and cerebral iron homeostasis in ferritin knock-out mice. Public Library of Science One 10, e0117435.
LV Z., JIANG H., XU H., SONG N. & XIE J. (2011): Increased iron levels correlate with the selective nigral dopaminergic neuron degeneration in Parkinson's disease. Journal of Neural Transmission (Vienna) 118, 361-369.
MANN G. (1894): Histological changes induced in sympathetic, motor, and sensory nerve cells by functional activity (preliminary note). Journal of Anatomy and Physiology 29, 100-108.
MARINESCO G. (1902): [On the presence of paranucleolar acidophilic corpuscles in the cells of the locus niger and the locus coeruleus. French.] Comptes Rendus de l'Académie des Sciences 135, 1000-1002.
MEDEIROS M.S., SCHUMACHER-SCHUH A., CARDOSO A.M., BOCHI G.V., BALDISSARELLI J., KEGLER A., SANTANA D., CHAVES C.M., SCHETINGER M.R., MORESCO R.N., RIEDER C.R. & FIGHERA M.R. (2016): Iron and oxidative stress in parkinson’s disease: an observational study of injury biomarkers. Public Library of Science one 11, e0146129.
MEGURO R., ASANO Y., ODAGIRI S., LI C., IWATSUKI H. & SHOUMURA K. (2007): Nonheme-iron histochemistry for light and electron microscopy: a historical, theoretical and technical review. Archives of Histology and Cytology 70, 1-19.
MIZUNO Y., HORI S., KAKIZUKA A. & OKAMOTO K. (2003): Vacuole-creating protein in neurodegenerative diseases in humans. Neuroscience Letters 343, 77-80.
MORI F., TANJI K., ODAGIRI S., TOYOSHIMA Y., YOSHIDA M., KAKITA A., TAKAHASHI H. & WAKABAYASHI K. (2012a): Autophagy-related proteins (p62, NBR1 and LC3) in intranuclear inclusions in neurodegenerative diseases. Neuroscience Letters 522, 134-138.
MORI F., TANJI K., TOYOSHIMA Y., YOSHIDA M., KAKITA A., TAKAHASHI H. & WAKABAYASHI K. (2012b): Optineurin immunoreactivity in neuronal nuclear inclusions of polyglutamine diseases (Huntington's, DRPLA, SCA2, SCA3) and intranuclear inclusion body disease. Acta Neuropathologica 123, 747-749.
NAIR-ROBERTS R.G., CHATELAIN-BADIE S.D., BENSON E., WHITE-COOPER H., BOLAM J.P. & UNGLESS M.A. (2008): Stereological estimates of dopaminergic, GABAergic and glutamatergic neurons in the ventral tegmental area, substantia nigra and retrorubral field in the rat. Neuroscience 152, 1024-1031.
NGUYEN-LEGROS J., BIZOT J., BOLESSE M. & PULICANI J.P. 1980: ["Diaminobenzidine black" as a new histochemical demonstration of exogenous iron (author's transl). French.]. Histochemistry 66, 239-244.
OAKLEY A.E., COLLINGWOOD J.F., DOBSON J., LOVE G., PERROTT H.R., EDWARDSON J.A., ELSTNER M. & MORRIS C.M. (2007): Individual dopaminergic neurons show raised iron levels in Parkinson disease. Neurology 68, 1820-1825.
ODAGIRI S., TANJI K., MORI F., KAKITA A., TAKAHASHI H., KAMITANI T. & WAKABAYASHI K. (2012): Immunohistochemical analysis of Marinesco bodies, using antibodies against proteins implicated in the ubiquitin–proteasome system, autophagy and aggresome formation. Neuropathology 32, 261–266.
OKAMOTO K. & HIRAI S. (1981): [Fine structures of Marinesco body and nuclear body in the substantia nigra. Japanese.] Rinsho Shinkeigaku [Clinical Neurology] 21, 781-789.
QIAN Z.M. & WANG Q. (1998): Expression of iron transport proteins and excessive iron accumulation in the brain in neurodegenerative disorders. Brain Research. Brain Research Reviews 27, 257-267.
RAMÓN Y CAJAL S. (1909): Histologie du systeme nerveus de l’homme et des vertebres. Vol I. Translated by L. Azoulay. Paris: Maloine.
RAMÓN Y CAJAL S. (1911): [Histology of the nervous system of humans and vertebrates. French.]. Vol. II. Paris: Maloine.
RONCORONI L. (1895): [On a new finding in the nucleus of nerve cells. Italian.]. Archivio di Psicologia, Neurologia e Psichiatria 16, 447-450.
SHAMOTO-NAGAI M., MARUYAMA W., YI H., AKAO Y., TRIBL F., GERLACH M., OSAWA T., RIEDERER P. & NAOI M. (2006): Neuromelanin induces oxidative stress in mitochondria through release of iron: mechanism behind the inhibition of 26S proteasome. Journal of Neural Transmission 113, 633-644.
SHINOBU L.A. & BEAL M.F. (1997): The role of oxidative processes and metal ions in aging and Alzheimer's disease. In: Metals and Oxidative Damage in Neurological Disorders (Ed Connor J.R.), pp. 237-275, New York: Springer Science Media.
SIAN-HULSMANN J., MANDEL S., YOUDIM M.B. & RIEDERER P. (2011): The relevance of iron in the pathogenesis of Parkinson's disease. Journal of Neurochemistry 118, 939-957.
SOFIC E., PAULUS W., JELLINGER K., RIEDERER P. & YOUDIM M.B. (1991): Selective increase of iron in substantia nigra zona compacta of parkinsonian brains. Journal of Neurochemistry 56, 978-982.
SUKHORUKOVA E.G., ALEKSEEVA O.S. & KORZHEVSKY D.E. (2014): Catecholaminergic neurons of mammalian brain and neuromelanin. Journal of Evolutionary Biochemistry and Physiology 50, 383-391.
SUKHORUKOVA E.G., GRIGORIEV I.P., KIRIK O.V., ALEKSEEVA O.S. & KORZHEVSKII D.E. (2013): Intranuclear localization of iron in neurons of mammalian brain. Journal of Evolutionary Biochemistry and Physiology 49, 370-372.
TAKAHASHI-FUJIGASAKI J. & FUJIGASAKI H. (2006): Histone deacetylase (HDAC) 4 involvement in both Lewy and Marinesco bodies. Neuropathology and Applied Neurobiology 32, 562-566.
TAN W., XUE-BIN C., TIAN Z., XIAO-WU C., PEI-PEI H., ZHI-BIN C. & BEI-SHA T. (2016): Effects of simvastatin on the expression of inducible nitric oxide synthase and brain-derived neurotrophic factor in a lipopolysaccharide-induced rat model of Parkinson disease. International Journal of Neuroscience 126, 278-286.
TSAKIRI E.N. & TROUGAKOS I.P. (2015): The amazing ubiquitin-proteasome system: structural components and implication in aging. International Review of Cell and Molecular Biology 314, 171-237.
VIDAL R., MIRAVALLE L., GAO X., BARBEITO A.G., BARAIBAR M.A., HEKMATYAR S.K., WIDEL M., BANSAL N., DELISLE M.B. & GHETTI B. (2008): Expression of a mutant form of the ferritin light chain gene induces neurodegeneration and iron overload in transgenic mice. Journal of Neuroscience 28, 60-67.
WANG J., JIANG H. & XIE J.X. (2007): Ferroportin1 and hephaestin are involved in the nigral iron accumulation of 6-OHDA-lesioned rats. European Journal of Neuroscience 25, 2766-2772.
WOULFE J., GRAY D., PRITCHET-PEJIC W., MUNOZ D.G. & CHRETIEN M. (2004): Intranuclear rodlets in the substantia nigra: interactions with Marinesco bodies, ubiquitin and promyelocytic leukemia protein. Journal of Neuropathology and Experimental Neurology 63, 1200–1207.
WOULFE J.M., HAMMOND R., RICHARDSON B., SOORIABALAN D., PARKS W., RIPPSTEIN P. & MUNOZ D.G. (2002): Reduction of neuronal intranuclear rodlets immunoreactive for tubulin and glucocorticoid receptor in Alzheimer’s disease. Brain Pathology 12, 300–307.
WOULFE J. & MUNOZ D. (2000): Tubulin immunoreactive neuronal intranuclear inclusions in the human brain. Neuropathology and Applied Neurobiology 26, 161–171.
WOULFE J.M., PRICHETT-PEJIC W., RIPPSTEIN P. & MUNOZ D.G. (2007): Promyelocytic leukaemia-immunoreactive neuronal intranuclear rodlets in the human brain. Neuropathology and Applied Neurobiology 33, 56-66.
WU Y. & BROSH R.M. JR. (2012): DNA helicase and helicase-nuclease enzymes with a conserved iron-sulfur cluster. Nucleic Acids Research 40, 4247-4260.
YOUDIM M.B., BEN-SHACHAR D. & RIEDERER P. (1991): Iron in brain function and dysfunction with emphasis on Parkinson's disease. European Neurology 31 (Suppl 2), 34-40.
YUEN P. & BAXTER D.W. (1963): The morphology of Marinesco bodies (paranucleolar corpuscles) in the melanin-pigmented nuclei of the brain-stem. Journal of Neurology, Neurosurgery, & Psychiatry 26, 178–183.
L. Zecca, T. Shima, A. Stroppolo, C. Goj, G. A. Battiston, R. Gerbasi, T. Sarna, H. M. Swartz. Interaction of neuromelanin and iron in substantia nigra and other areas of human brain. Neuroscience Vol. 73, No. 2, pp. 407-415, 1996
ZECCA L., STROPPOLO A., GATTI A., TAMPELLINI D., TOSCANI M., GALLORINI M., GIAVERI G., AROSIO P., SANTAMBROGIO P., FARIELLO R.G., KARATEKIN E., KLEINMAN M.H., TURRO N., HORNYKIEWICZ O. & ZUCCA F.A. (2004): The role of iron and copper molecules in the neuronal vulnerability of locus coeruleus and substantia nigra during aging. Proceedings of the National Academy of Sciences of the United States of America 101, 9843-9848.
ZUCCA FA, SEGURA-AGUILAR J, FERRARI E, MUÑOZ P, PARIS I, SULZER D, SARNA, T., CASELLA, L. & ZECCA, L. (2015): Interactions of iron, dopamine and neuromelanin pathways in brain aging and Parkinson’s disease. Progress in Neurobiology 155, 96-119.