Matrix metalloproteinase 9, MMP-9 is an extracellularly operating enzyme that has been demonstrated as an important regulatory molecule in control of synaptic plasticity, learning and memory. Either genetic or pharmacological inhibition of MMP-9 impairs late phase of long-term potentiation at various pathways, as well as appetitive and spatial memory formation, although aversive learning remains apparently intact in MMP-9 KO mice. MMP-9 is locally translated and released from the excitatory synapses in response to neuronal activity. Extrasynaptic MMP-9 is required for growth and maturation of the dendritic spines to accumulate and immobilize AMPA receptors, making the excitatory synapses more efficacious. Animal studies have implicated MMP-9 in such neuropsychiatric conditions, as e.g., epileptogenesis, autism spectrum disorders, development of addiction, and depression. In humans, MMP-9 appears to contribute to epilepsy, alcohol addiction, Fragile X Syndrome, schizophrenia and bipolar disorder. In aggregate, all those conditions may be considered as relying on alterations of dendritic spines/excitatory synapses and thus understanding the role played by MMP-9 in the synaptic plasticity may allow to elucidate the underpinnings of major neuropsychiatric disorders.
Synaptic plasticity: A concept
The term “synaptic plasticity” has been introduced by Jerzy Konorski from the Nencki Institute in 1948, in his book: “Conditioned reflexes and neuron organization”. The idea was based on long-standing concept, originally proposed by Ramon y Cajal, as Kandel et al. (2014) have recently recalled: “…the cellular connectionist approach, which derived from Cajal’s idea that memory is stored as an anatomical change in the strength of synaptic connections (Cajal, 1894). (In 1948 Konorski renamed Cajal’s idea synaptic plasticity [the ability of neurons to modulate the strength of their synapses as a result of use (Konorski, 1948)].”
For a long time, starting from 70-ties and discovery of long-term potentiation of synaptic efficacy (LTP), the “synaptic plasticity” term was mainly used by electrophysiologists. However, nowadays, both anatomical, physiological and even its molecular meanings are widely accepted. In a broad sense, synaptic plasticity refers to modifications of synaptic efficacy within vast neuronal network of the brain that processes in malleable way incoming information to produce behaviors that change because of the associated past stimuli and their reinforcing value.
Synaptic plasticity has thus become a useful description for underpinnings of such physiological phenomena as learning and memory, as well as critical periods of cortical postnatal development, regenerative response to neuronal injuries and a host of neuropsychiatric conditions, including epileptogenesis, development of addictive behaviors, schizophrenia, depression or autism spectrum disorders, to name some prominent examples.
Especially noticeable is plasticity of excitatory synapses and harboring them dendritic spines. Their plasticity apparently underlies LTP and LTD (long-term depression) of synaptic efficacy, as well as silencing/unsilencing of synapses and accompanying changes in AMPA/NMDA receptor ratio, all detected by electrophysiological means (Malenka, Bear, 2004). At the structural level, parallel phenomena include changes in the dendritic spine density, shape and size. The spines are typically classified as either small or thin of filopodial or stubby or mushroom (Engert, Bonhoeffer, 1999; Matsuzaki et al., 2004; Sala, Segal, 2014; Nishiyama, Yasuda, 2015). In general, the mushroom ones are believed to be the most mature, with greatest numbers of glutamate receptors and high AMPA/NMDA receptor ratio. As these receptors are located at the region of so called postsynaptic density (PSD), easily discerned by the electron microscopy approach, the size of PSD correlates with synaptic efficacy, along the area/volume of the spine head. Furthermore, larger, more efficacious synapses are characterized by specific protein content, defining their molecular signature.
MMP-9: an extracellular protease
MMP-9, matrix metalloproteinase-9 is a protease that operates predominantly extracellularly. It belongs to a larger family of enzymes (metalloproteinases) that together with other similar molecules form an abundant class of metzincins (including also astacins, ADAMs, ADAMTSs, etc., Rivera et al., 2010; Vandooren et al., 2013). To make the researcher’s life harder, all those enzymes are somewhat similar in structure, although rapidly evolving, what in aggregate makes very difficult to obtain specific antibodies towards individual members of the group. Furthermore, the enzymes are quite promiscuous against the substrates, cleaving in a tube many different proteins, leading to a very serious problem of finding specific inhibitors of individual enzymes. Finally, the levels of MMP-9 in the "naive" brain are dramatically low and they markedly increase only upon appropriate stimulation, however, of various nature, ranging from inflammation to synaptic plasticity. Moreover, since activation of MMPs usually requires their partial cleavage to remove a propeptide that covers the enzymatic active site, the enzymes work in cascades with one activating another, to activate yet another, and so on. This means that always we have to deal with and to distinguish between several enzymatic activities in a given cellular response. Thus, it has to be said with very strong emphasis that studying MMP-9 (as well as its cognates) demands to use simultaneously a variety of approaches, e.g., to detect mRNA, protein and enzymatic activity in parallel, all of those by various techniques, to be relatively assured that indeed MMP-9 is present and functional (see Vafadari et al., 2016).
MMP-9 in synaptic plasticity, learning and memory
Probably the first indication for a possible role of MMP-9 in brain physiology comes from our studies on kainate-evoked neuronal cell loss and epileptogenesis (Szklarczyk et al., 2002). Until then, multiple studies implicated MMP-9 in pro-neurodegenerative, pathological responses, easily associated with excessive, tissue-detrimental proteolysis (Yong et al., 1998; Yong, 2005, Lo et al., 2002). Since peripheral injection of kainate produces CA1 and CA3 hippocampal cell loss, along, e.g., with similar damage of the entorhinal cortex (EC), observing enhanced MMP-9 after kainate came as no surprise. Nonetheless, it was very unexpected to see selective MMP-9 increase in the hippocampal dentate gyrus (DG), as opposed to CA1 and CA3, where very low levels of MMP-9 were noted (Szklarczyk et al., 2002). This finding could, however, be explained by neuronal and synaptic plasticity that occurs specifically in this brain area, as granule cells of DG lose both their input from EC and output (CA3) and, in result, produce aberrant synaptogenesis on themselves (that may contribute to subsequent epileptogenesis, Zagulska-Szymczak et al., 2001). Even more surprising was our discovery that increases in MMP-9 in dendritic tree area of DG concern not only the protein and enzymatic activity, but also mRNA, suggestive of its translocation towards synapses that were undergoing plastic reorganization. In result, we have put forward a hypothesis of possible role of MMP-9 in dendritic remodeling and synaptic plasticity, as well as of local, dendritic/synaptic translation of MMP-9 during plasticity (Szklarczyk et al., 2002).
Experimental support for this hypothesis was published a few years later. In 2006, Nagy et al., as well as Meighan et al. demonstrated by various means that MMP-9 was indispensable for late (over half an hour) phase of hippocampal (CA3-CA1) LTP, as well as for hippocampal learning and memory (contextual fear conditioning, water maze), as shown unequivocally with MMP-9 knockout (KO) mice, along other less specific means. Furthermore, increases in MMP-9 protein and enzymatic activity levels were also demonstrated (Nagy et al., 2006; Meighan et al., 2006). Soon thereafter, these observations were extended to other experimental systems of LTP, learning and memory that all involved the hippocampus (Bozdagi et al., 2007; Nagy et al., 2007; Okulski et al., 2007; Wright et al., 2006; 2007; Conant et al., 2010; Wojtowicz, Mozrzymas, 2010; Huntley, 2012; Tsilibary et al., 2014). In contrast, formation of aversive memories that relies on Lateral Amygdala (LaA) does not require MMP-9 activity (Nagy et al., 2006; Knapska et al., 2013), and LTP might be evoked with no alterations on the external capsule-LaA pathway, even when MMP-9 is missing (Gorkiewicz et al., 2014). On the other hand, appetitive learning and memory, as well as LTP from LaA to Basal Amygdala (BA) and from BA to medial Central Amygdala (mCeA) misses the lasting phase (over 20 min), when MMP-9 activity is not available (Knapska et al., 2013; Gorkiewicz et al., 2014). Therefore, it is of note that MMP-9 is not universally mandatory for synaptic plasticity, learning and memory. Nevertheless, activity of this molecule is an obligatory component for specific forms of those phenomena, especially in the hippocampus. An important role of MMP-9 in synaptic function underlying postnatal and even adult cortical plasticity has also been shown for the visual and somatosensory cortex (Szklarczyk, Kaczmarek, 2005; Spolidoro et al., 2012; , Kaliszewska et al., 2012; Verslegers et al., 2013; Kelly et al., 2015). Similarly, requirement for MMP-9 has also been demonstrated for chemical LTP (cLTP) in hippocampal cultures (Niedringhaus et al., 2012; 2013: Szepesi et al., 2013; 2014).
Local translation of MMP-9
We have also followed the hypothesis of local translation of MMP-9 at/around activated excitatory synapses. It should be noted that our other studies clearly pointed to MMP-9 presence on dendritic spines, and around postsynaptic areas of excitatory synapses, selectively, as opposed to non-detectable MMP-9 at either presynaptic domains or GABA-ergic synapses (Wilczynski et al., 2008; Gawlak et al., 2009). Konopacki et al. (2007), using fluorescent in situ hybridization combined with immunofluorescent protein detection, reported on a patchy MMP-9 mRNA accumulation in DG dendrites in response to kainate treatment. This result reinforced the idea of MMP-9 mRNA being translocated, after kainate, towards excitatory synapses. Dziembowska et al. (2012) and Janusz et al. (2013) provided a number of experimental data clearly showing that indeed MMP-9 mRNA can undergo local synaptic translation to produce the protein, after activation of excitatory synapses. These experiments have also revealed that MMP-9 production, release and synaptic availability after synaptic activation occurs within a few minutes following treatment with glutamate (see also Michaluk et al., 2007).
MMP-9 in structural and functional synaptic plasticity: The mechanisms
The evidence for pivotal role of MMP-9 in structural plasticity of dendritic spines comes from hippocampal cultures and slices, as well as the brain in vivo (see Dziembowska and Wlodarczyk, 2012; Stawarski et al., 2014; Vafadari et al., 2016 for review). Two major observations have been made. First, excessive availability of MMP-9 produces elongation and thinning of the spines (Bilousova et al., 2009; Michaluk et al., 2011). On the other hand, physiologically and locally available MMP-9 evokes conversion of small spines to larger, more efficacious mushroom ones (Wang et al., 2008; Szepesi et al., 2014). We have recently explained this apparent paradox, by finding that the full function of MMP-9 requires first its activity, followed by subsequent inhibition, exerted physiologically by TIMP-1 (tissue inhibitor of matrix metallproteinases-1, Maganowska, Gorkiewicz et al., submitted).
We have also found that excessive MMP-9 in transgenic rats with neuronal overexpression of the enzyme (Wilczynski et al., 2008) results in higher, than in the wild-type rats, proportion of silent synapses and lower AMPA/NMDA receptor ratio, along with impaired LTP. Treatment with MMP inhibitors in those transgenics normalized (i.e., enhanced) LTP as well as unsilenced the synapses and finally resulted in increased AMPA/NMDA receptor ratio (Maganowska, Gorkiewicz et al., submitted).
Using hippocampal cultures subjected to cLTP we have found that this form of synaptic plasticity correlates with growth of small spines into larger mushroom ones, concomitantly with synaptic accumulation of GluA1 AMPA receptors that are at same time less mobile at the synapses. All these major attributes of synaptic plasticity were lost when cLTP treatment was carried under MMP inhibition, i.e., neither spine growth, nor GluA1 accumulation and immobilization at the synapses could be observed under such conditions (Szepesi et al., 2014).
In vivo, Sidhu et al. (2014) observed that MMP-9 KO mice display larger spine head areas in the hippocampus at 1-2 weeks postnatally (later on, as the authors found, this value becomes the same as in the wild type animals). Interestingly, Aujla and Huntley (2014) found that levels of MMP-9 peak in the hippocampus more or less at the same time. Murase et al. (2014) showed that MMP-9 KO mice have unaltered spine density in the hippocampus of adult animals, however, there is increase in proportion of mushroom spine on the expense of thin ones. Kelly et al. (2015), while studying ocular dominance plasticity in the mouse visual cortex, observed no change in the morphology of existing dendritic spines in MMP-9 KO, however, spine dynamics were altered and KO mice showed increased turnover of dendritic spines over a period of 2 days. Fragkouli et al. (2012) constructed mice overexpressing MMP-9 and reported on increased spine density in the hippocampus and somatosensory cortex after behavioral training of adult animals.
Synaptic targets of MMP-9: The misleading name - MMP-9 does not affect ECM but cleaves synaptic CAMs instead
It may appear, intuitively, obvious that MMP-9 should cleave components of the extracellular matrix (ECM) surrounding the synapses. In fact, Tsien (2013) has proposed that such a cleavage may relieve the synapses/dendritic spines from local environmental constraints limiting their growth, any by this virtue allowing them to undergo plastic changes supporting learning and memory. It should be noted that disruption of ECM may indeed affect synaptic plasticity (Frishknecht et al., 2009; Dityatev, Rusakov, 2011; Soleman et al., 2013; Tamura et al., 2013). However, although possible role in MMP-9 in ECM remodeling has been suggested by studies on the cerebellum, no clear MMP-9 substrate has emerged, and in fact we have failed to demonstrate that MMP-9 cleaves a suspected substrate, tenascin-C (Foscarin et al., 2011; Stamenkovic et al., in press and unpublished data). Similarly, it remains as an attractive, though unproven possibility that MMP-9 might cleave CD44 that may anchor hyaluronic acid-based ECM at the neuronal cell membrane.
Notably, treatment with excessive exogenous MMP-9 did not produce any gross alteration of ECM in hippocampal cultures (Michaluk et al., 2009). Furthermore, no ECM proteins surrounding synapses have been identified as MMP-9 substrates. In fact, most of such substrates belong to the category of cell adhesion molecules (CAMs, Bajor, Kaczmarek, 2013; Conant et al., 2015; Shinoe, Goda, 2015). Even more interestingly, all of them are CAMs that might be found located postsynaptically. The group includes: b-dystroglycan, ICAM-5, neuroligin-1, SynCAM2 (synaptic cell adhesion molecule-2 also known as necl-3) and nectin-3 (Michaluk et al. 2007; Tian et al., 2007; Peixoto et al., 2012; Kelly et al., 2014; van der Kooij et al., 2014; Stawarski et al., 2014b). Most importantly, all of those proteins may form trans-synaptic adhesive apparatus with their presynaptic binding partners (b-dystroglycan and neuroligin-1 with neurexins, nectin-3 with nectin-1, ICAM-5 with ICAM-5, SynCAM2 with SynCam1). Other neuronal MMP-9 substrates identified to date are collapsin response mediator protein-2 (CRMP-2, Bajor et al., 2012), NGF (Bruno Cuello, 2006) and pro-BDNF (Mizoguchi et al., 2011).
Considering trans-synaptic adhesive apparatus as a major MMP-9 target and taking into account other aforementioned information, one may suggest that following glutamate stimulation, especially by NMDA receptors, MMP-9 is released from small dendritic spines around postsynaptic domains of excitatory synapses. Next, MMP-9 destabilizes synaptic structure by breaking trans-synaptic connections through limited cleavage of postsynaptically originating proteins bound to their presynaptic partners. This way, the postsynapse and its dendritic spine carrier are allowed to expand and maybe search for a new presynaptic partner. As soon as MMP-9 is inhibited by endogenous TIMP-1, the pre-postsynaptic connection is (re-)established, however in a modified, possible more efficacious form. Such a scenario is in a perfect agreement with the available experimental data and provides a good explanation for MMP-9 pivotal role in the synaptic plasticity, learning and memory.
However, the molecular mechanisms delineated above, although plausible are not proven yet. Thus, either alternative or complementary modes of MMP-9 function in synaptic plasticity have to be considered. Especially intriguing is partial cleavage of pro-BDNF to produce its mature form (Mizoguchi et al., 2011) as well repeatedly described mediation of MMP-9 synaptic effects via integrins, in particular integrin b1 (Nagy et al., 2006; Wang et al., 2002; Michaluk et al., 2011; Niedringhaus et al., 2012)
MMP-9 as executor of c-Fos function in synaptic plasticity, learning and memory?
Our research interest in MMP-9 has been spurred by the previous studies on c-Fos. In the late eighties gene expression in learning was discovered, when rapid and transient c-fos mRNA accumulation was demonstrated in the brain following glutamate injection, induction of LTP and behavioral training (Kaczmarek et al., 1988; Maleeva et al., 1989; 1990; Kaczmarek and Nikolajew 1990; Tischmeyer et al., 1990, Nikolaev at al., 1991; 1992 a,b; Bialy et al., 1992; Kaczmarek, 1992, 1993a,b). A plethora of other studies confirmed and extended those findings to multiple instances of plasticity, as well as learning and memory phenomena (Kaczmarek, 2002). Importantly elevated c-fos expression was observed only when the animals were learning new task, but not when vigorously performing already learned behavior (Nikolaev et al., 1992b).
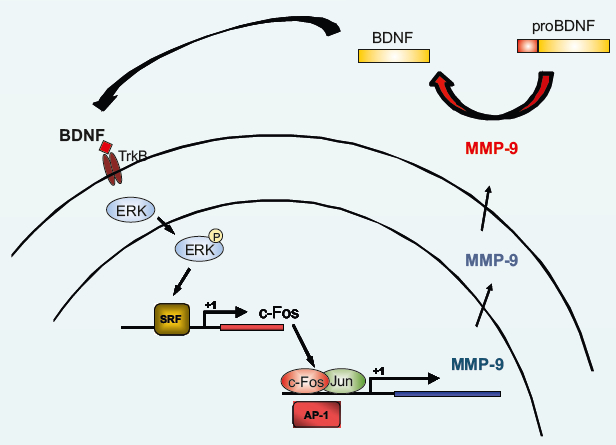
To get an insight into mechanisms controlled by c-Fos protein that could explain its role in the brain, we turned to its only recognized molecular function, i.e., being active as transcriptional regulator, as a component of AP-1 transcription factor. After extensive search of c-Fos/AP-1 regulated genes in activated neurons, we have provided extensive evidence for TIMP-1 being such a target (Jaworski et al., 1999). This, in turn, shifted our attention to MMP-9 as being targeted by TIMP-1. Interestingly, numerous evidence outside the nervous system suggested that also MMP-9 might be regulated by c-Fos/AP-1 (Kaczmarek et al., 2002). In fact, we have also shown that this is the case also in the brain, after behavioral training of fear conditioning and in BDNF-activated neurons in culture (Ganguly et al., 2013; Kuzniewska et al., 2013). I would even dare to say that TIMP-1 and MMP-9 are the best documented c-Fos/AP-1 gene targets in stimulated neurons. Thus, considering very abundant, although still largely circumstantial, evidence implicating c-Fos in synaptic plasticity in learning, one may hypothesize that c-Fos role in these phenomena might be executed via MMP-9 and TIMP-1. A following molecular scenario might be even considered here. During learning experience, glutamate activates NMDA receptors to release MMP-9 and TIMP-1 to control the synaptic plasticity as described above. Since both proteins are released outside the cell and cannot be recuperated, there is a need to replenish them. MMP-9 activity, e.g., by converting pro-BDNF to its mature form (mBDNF) produces a signal that through TrkB receptors and ERK kinases is delivered to SRF transcription factor that is the major upregulator of c-fos gene expression in activated neurons. Next, the protein product, c-Fos in a form of AP-1, enhances transcription of MMP-9 gene.
MMP-9 in neuropsychiatric disorders: A case of aberrant synaptic plasticity?
Besides being pivotal for physiological synaptic plasticity, as documented above, MMP-9 has also been implicated in aberrant plasticity that may contribute to a variety of neuropsychiatric conditions (Reinhard et al., 2015; Vafadari et al., 2016). The evidence for such a statement comes from both the studies on experimental animals, as well as human tissue. A particular strong case is presented here by epileptogenesis (Wilczynski et al., 2008; and for review: Lukasiuk et al., 2011; Vafadari et al., 2016). Similarly, addiction to substances of abuse was linked to MMP-9 (Smith et al., 2014; 2015; Mash et al., 2007; Brown et al., 2007; 2008; Conant et al., 2011; Samochowiec et al., 2010; Mizoguchi et al., 2007a; b). Furthermore, Lepeta and Kaczmarek (2015), reviewing the existing literature, have found a number of findings highly suggestive of a role of MMP-9 in schizophrenia. Finally, functional role of MMP-9 has been demonstrated in Fragile X Syndrome (FXS) that offers a very interesting example of autistic conditions derived from a single gene mutation (Fragile X Mental Retardation 1 Protein, FMRP). Bilousova et al. (2009) was the first to show that FMRP KO mice displayed increased MMP-9 activity and then Janusz et al. (2013) found that local translation of MMP-9 is FMRP-controlled. Similarly, Gkogkas et al. (2014) found that the eukaryotic translation initiation factor P-eIF4E and MMP-9 expression were both elevated in the brains of human FXS patients and in FMRP deficient mice. Furthermore, Bilousova et al. (2009) observed dendritic spine elongation in neuronal cultures that were derived from FMRP KO, a phenomenon that could be normalized by application of minocycline, which inhibited the enzymatic activity of MMP-9. Minocycline treatment also reduced anxiety in FMRP knockout mice (Bilousova et al., 2009) and reversed the deficit in ultrasonic vocalizations (Rotschafer et al., 2012). Finally, Sidhu et al. (2014) crossed MMP-9 KO mice with FMRP KO mice, to alleviate all of the major symptoms of FXS that were observed in FMRP KO. These results strongly supported the consideration of minocycline as a treatment for FXS. Indeed, several clinical studies that have been conducted, offered promising results (Paribello et al., 2010; Leigh et al., 2013; Siller and Broadie, 2012; Dziembowska et al., 2013; for review, see Hagerman and Polussa, 2015).
Figure 1. Schematic representation of the proposed feedback loop between c-Fos and MMP-9. When MMP-9 is released and activated in response to synaptic stimulation, it may cleave pro-BDNF to produce mature BDNF that in turn activates TrkB receptor to signal through ERK kinases to the cell nucleus to stimulate SRF transcription factor controlling c-fos gene expression. Finally, c-Fos (together with Jun protein) acts as AP-1 transcription factor turning on MMP-9 gene transcription (see Kuzniewska et al., 2013 for details).
Concluding remarks
The evidence for a role of MMP-9 in synaptic plasticity appears very compelling, indeed. It is derived from studies documenting enhanced MMP-9 levels (mRNA, protein and enzymatic activity) unequivocally in response to stimuli that evoke plasticity and, moreover, those increases occur in/around stimulated excitatory synapses. Furthermore, blocking of MMP-9, including using the most specific, gene KO approach impairs the plasticity. Finally, enhancement of MMP-9 was also shown to affect synaptic plasticity. These data seem to explain well the role of the enzyme in learning and memory, and may be also considered as an justification for MMP-9 dysfunctions in major neuropsychiatric disorders. Nevertheless, in the latter case, MMP-9 involvement in neuroinflammation that clearly contributes to these disorders, cannot be overlooked, as alternative to the synaptic plasticity, explanation (see Vafadari et al., 2016).
| Attachment | Size |
|---|---|
| 352.98 KB |
Aujla P. K. and Huntley G. W. (2014) Early postnatal expression and localization of matrix metalloproteinases-2 and -9 during establishment of rat hippocampal synaptic circuitry. J. Comp. Neurol. 522, 1249-1263.
Bajor M. and Kaczmarek L. (2013) Proteolytic Remodeling of the Synaptic Cell Adhesion Molecules (CAMs) by Metzincins in Synaptic Plasticity. Neurochem. Res. 38, 1113-1121.
Bajor M., Michaluk P., Gulyassy P., Kekesi A. K., Juhasz G. and Kaczmarek L. (2012) Synaptic cell adhesion molecule-2 and collapsin response mediator protein-2 are novel members of the matrix metalloproteinase-9 degradome. J. Neurochem. 122, 775-788.
Bialy M., Nikolaev E., Beck J., Kaczmarek L., (1992) Delayed c-fos expression in sensory cortex following sexual learning in male rats. Mol. Brain Res., 14: 352-356.
Bilousova T. V., Dansie L., Ngo M., Aye J., Charles J. R., Ethell D. W. and Ethell, I. M. (2009) Minocycline promotes dendritic spine maturation and improves behavioural performance in the fragile X mouse model. J. Med. Genet. 46, 94-102.
Bozdagi O., Nagy V., Kwei K. T. and Huntley G. W. (2007) In vivo roles for matrix metalloproteinase-9 in mature hippocampal synaptic physiology and plasticity. J. Neurophysiol. 98, 334-344.
Brown T. E., Forquer M. R., Cocking D. L., Jansen H. T., Harding J. W. and Sorg B. A. (2007) Role of matrix metalloproteinases in the acquisition and reconsolidation of cocaine-induced conditioned place preference. Learn. Mem. 14, 214-223.
Brown T. E., Forquer M. R., Harding J. W., Wright J. W. and Sorg B. A. (2008) Increase in matrix metalloproteinase-9 levels in the rat medial prefrontal cortex after cocaine reinstatement of conditioned place preference. Synapse 62, 886-889.
Bruno M. A. and Cuello A. C. (2006) Activity-dependent release of precursor nerve growth factor, conversion to mature nerve growth factor, and its degradation by a protease cascade. Proc. Natl Acad. Sci. USA. 103, 6735-6740.
Cajal, S.R. (1894) La fine structure des centres nerveux. Proc. R. Soc. Lond.; 55: 444–468.
Conant K., Allen M., Lim S. T. (2015) Activity Dependent CAM cleavage and Neurotransmission. Front. Cell. Neurosci. 9, 305.
Conant K., Lonskaya I., Szklarczyk A., Krall, C. Steiner J., Maguire-Zeiss K. and Lim S. T. (2011) Methamphetamine-associated cleavage of the synaptic adhesion molecule intercellular adhesion molecule-5. J. Neurochem. 118, 521-532.
Conant K., Wang Y., Szklarczyk A., Dudak A., Mattson M. P. and Lim S. T. (2010) Matrix metalloproteinase-dependent shedding of intercellular adhesion molecule-5 occurs with long-term potentiation. Neuroscience 166, 508-521.
Dityatev A, Rusakov DA. (2011) Molecular signals of plasticity at the tetrapartite synapse. Curr Opin Neurobiol. 21: 353-359.
Dziembowska M., Milek J., Janusz A., Rejmak E., Romanowska E., Gorkiewicz T., Tiron A., Bramham C. R. and Kaczmarek L. (2012) Activity-dependent local translation of matrix metalloproteinase-9. J. Neurosci. 32, 14538-14547.
Dziembowska M and Wlodarczyk J. (2012) MMP9: a novel function in synaptic plasticity. Int. J. Biochem. Cell Biol. 44, 709-713
Dziembowska M., Pretto D. I., Janusz A., Kaczmarek L., Leigh M. J., Gabriel N., Durbin-Johnson B., Hagerman R. J. and Tassone F. (2013) High MMP-9 activity levels in fragile X syndrome are lowered by minocycline. Am. J. Med. Genet. A. 161A, 1897-1903.
Engert F. and Bonhoeffer T. (1999) Dendritic spine changes associated with hippocampal long-term synaptic plasticity. Nature 399, 66-70.
Foscarin S, Ponchione D, Pajaj E, Leto K, Gawlak M, Wilczynski GM, Rossi F, Carulli D. (2011) Experience-dependent plasticity and modulation of growth regulatory molecules at central synapses. PLoS One. 6: e16666.
Fragkouli A., Papatheodoropoulos C., Georgopoulos S., Stamatakis A., Stylianopoulou F., Tsilibary E. C. and Tzinia A. K. (2012) Enhanced neuronal plasticity and elevated endogenous sAPPalpha levels in mice over-expressing MMP9. J. Neurochem. 121, 239-251.
Frischknecht R, Heine M, Perrais D, Seidenbecher CI, Choquet D, Gundelfinger ED. (2009) Brain extracellular matrix affects AMPA receptor lateral mobility and short-term synaptic plasticity. Nat Neurosci. 12: 897-904.
Ganguly K., Rejmak E., Mikosz M., Nikolaev E., Knapska E. and Kaczmarek L. (2013) Matrix metalloproteinase (MMP) 9 transcription in mouse brain induced by fear learning. J. Biol. Chem. 288, 20978-20991.
Gawlak M., Gorkiewicz T., Gorlewicz A., Konopacki F. A., Kaczmarek L. and Wilczynski G. M. (2009) High resolution In situ zymography reveals matrix metalloproteinase activity at glutamatergic synapses. Neuroscience 158, 167-176.
Gkogkas C. G., Khoutorsky A., Cao R., Jafarnejad S. M, Prager-Khoutorsky M,, Giannakas N., Kaminari A., Fragkouli A., Nader K., Price T. J., Konicek B. W., Graff J. R., Tzinia A. K., Lacaille J. C. and Sonenberg N. (2014) Pharmacogenetic inhibition of eIF4E-dependent Mmp9 mRNA translation reverses fragile X syndrome-like phenotypes. Cell. Rep. 9, 1742-1755.
Gorkiewicz T., Balcerzyk M., Kaczmarek L. and Knapska E. (2015) Matrix metalloproteinase 9 (MMP-9) is indispensable for long term potentiation in the central and basal but not in the lateral nucleus of the amygdala. Front. Cell Neurosci. 9, 73.
Hagerman R. J. and Polussa J. (2015) Treatment of the psychiatric problems associated with fragile X syndrome. Curr. Opin. Psychiatry 28, 107-112.
Huntley G. W. (2012) Synaptic circuit remodelling by matrix metalloproteinases in health and disease. Nat. Rev. Neurosci. 13, 743-757.
Janusz A., Milek J., Perycz M., Pacini L., Bagni C., Kaczmarek L. and Dziembowska M. (2013) The Fragile X mental retardation protein regulates matrix metalloproteinase 9 mRNA at synapses. J. Neurosci. 33, 18234-18241.
Kaczmarek L., Siedlecki J.A., Danysz W., (1988) Protooncogene c-fos induction in rat hippocampus. Mol. Brain Res., 3: 183-186.
Kaczmarek L., (1992) Expression of c-fos and other genes encoding transcription factors in long term potentiation. Behav. Neural. Biol., 57: 263-266.
Kaczmarek L., (1993) Molecular biology of vertebrate learning: is c-fos a new beginning? J. Neurosci. Res., 34: 377-381.
Kaczmarek L., (1993) L-glutamate-driven gene expression in learning. Acta Neurobiol. Exp., 53: 187-196.
Kaczmarek L., (2002) c-Fos in learning: Beyond the mapping of neuronal activity. In: Handbook of Chemical Neuroanatomy, vol. 19: Immediate early genes and inducible transcription factors in mapping of the central nervous system function and dysfunction. Kaczmarek L., Robertson H.A. (eds.). Elsevier, Amsterdam, Boston, London, New York, Oxford, Paris, San Diego, San Francisco, Singapore, Sydney, Tokyo, pp. 189-216.
Kaczmarek L., Lapinska-Dzwonek J. and Szymczak S. (2002) Matrix metalloproteinases in the adult brain physiology: a link between c-Fos, AP-1 and remodeling of neuronal connections? EMBO. J. 21, 6643-6648.
Kaczmarek L., Nikolajew E., (1990) c-fos protooncogene expression and neuronal plasticity. Acta Neurobiol. Exp., 50: 173-179.
Kaliszewska A., Bijata M., Kaczmarek L. and Kossut M. (2012) Experience-dependent plasticity of the barrel cortex in mice observed with 2-DG brain mapping and c-Fos: effects of MMP-9 KO. Cereb. Cortex 22, 2160-2170.
Kandel ER, Dudai Y, Mayford MR. (2014) The molecular and systems biology of memory. Cell. 157: 163-186.
Kelly, E. A., Tremblay, M. E., Gahmberg, C. G., Tian, L. and Majewska, A. K. (2014) Subcellular localization of intercellular adhesion molecule-5 (Telencephalin) in the visual cortex is not developmentally regulated in the absence of Matrix Metalloproteinase-9. J. Comp. Neurol. 522, 676-688.
Kelly EA, Russo AS, Jackson CD, Lamantia CE, Majewska AK. (2015) Proteolytic regulation of synaptic plasticity in the mouse primary visual cortex: analysis of matrix metalloproteinase 9 deficient mice. Front Cell Neurosci. Sep 22;9:369.
Knapska E, Lioudyno V, Kiryk A, Mikosz M, Górkiewicz T, Michaluk P, Gawlak M, Chaturvedi M, Mochol G, Balcerzyk M, Wojcik DK, Wilczynski GM, Kaczmarek L. (2013) Reward Learning Requires Activity of Matrix Metalloproteinase-9 in the Central Amygdala. J. Neurosci. 33, 14591-14600.
Konopacki F.A., Rylski M., Wilczek E., Amborska R., Detka D., Kaczmarek L., Wilczyński G.M. (2007) Synaptic localization of seizure-induced matrix metalloproteinase-9 mRNA. Neuroscience, 150: 31-39.
Konorski, J. (1948) Conditioned reflexes and neuronal organization. Cambridge University Press, New York.
Kuzniewska B., Rejmak E., Malik A. R., Jaworski J., Kaczmarek L. and Kalita K. (2013) Brain-derived neurotrophic factor induces matrix metalloproteinase 9 expression in neurons via the serum response factor/c-Fos pathway. Mol. Cell. Biol. 33, 2149-2162.
Leigh M. J. S., Nguyen D. V., Mu Y. et al. (2013) A Randomized Double-Blind, Placebo-Controlled Trial of Minocycline in Children and Adolescents with Fragile X Syndrome. J. Dev. Behav. Pediatr. 34, 147-155.
Lepeta K. and Kaczmarek L. (2015) Matrix Metalloproteinase-9 as a Novel Player in Synaptic Plasticity and Schizophrenia. Schizophr. Bull. 41, 1003-9.
Lo EH, Wang X, Cuzner ML. (2002) Extracellular proteolysis in brain injury and inflammation: role for plasminogen activators and matrix metalloproteinases. J Neurosci Res. 69: 1-9.
Lukasiuk K., Wilczynski G. M. and Kaczmarek L. (2011) Extracellular proteases in epilepsy. Epilepsy Res. 96, 191-206.
Mash D. C., Ffrench-Mullen J., Adi N., Qin Y. J., Buck A. and Pablo J. (2007) Gene expression in human hippocampus from cocaine abusers identifies genes which regulate extracellular matrix remodeling. Plos One 2: e1187.
Maleeva NE, Ivolgina GL, Anokhin KV, Limborskaia SA. (1989) [Analysis of the expression of the c-fos proto-oncogene in the rat cerebral cortex during learning]. Genetika. 25: 1119-1121.
Maleeva NE, Bikbulatova LS, Ivolgina GL, Anokhin KV, Limborskaia SA, Kruglikov RI. (1990) [Activation of the c-fos proto-oncogene in different structures of the rat brain during training and pseudoconditioning]. Dokl Akad Nauk SSSR. 31: 762-764.
Malenka R. C. and Bear M. F. (2004) LTP and LTD: An embarrassment of riches. Neuron 44, 5-21.
Matsuzaki M., Honkura N., Ellis-Davies G. C. R. and Kasai H. (2004) Structural basis of long-term potentiation in single dendritic spines. Nature 429, 761-766.
Meighan S. E., Meighan P. C., Choudhury P., Davis C. J., Olson M. L., Zornes P. A., Wright J. W. and Harding J. W. (2006) Effects of extracellular matrix-degrading proteases matrix metalloproteinases 3 and 9 on spatial learning and synaptic plasticity. J. Neurochem. 96, 1227-1241.
Michaluk P., Kolodziej L., Mioduszewska B., Wilczynski G. M., Dzwonek J., Jaworski J., Gorecki D. C., Ottersen O. P. and Kaczmarek L. (2007) beta-dystroglycan as a target for MMP-9, in response to enhanced neuronal activity. J. Biol. Chem. 282, 16036-16041.
Michaluk P., Mikasova L., Groc L., Frischknecht R., Choquet D., Kaczmarek L. (2009) Matrix metalloproteinase-9 controls NMDA receptor surface diffusion through integrin beta1 signaling. J Neurosci. 29, 6007-6012
Michaluk P, Wawrzyniak M, Alot P, Szczot M, Wyrembek P, Mercik K, Medvedev N, Wilczek E, De Roo M, Zuschratter W, Muller D, Wilczynski GM, Mozrzymas JW, Stewart MG, Kaczmarek L, Wlodarczyk J. (2011) Influence of matrix metalloproteinase MMP-9 on dendritic spine morphology. J. Cell Sci. 124, 3369-3380.
Mizoguchi H., Nakade J, Tachibana M, Ibi D, Someya E, Koike H, Kamei H, Nabeshima T, Itohara S, Takuma K, Sawada M, Sato J, Yamada K. (2011) Matrix metalloproteinase-9 contributes to kindled seizure development in pentylenetetrazole-treated mice by converting pro-BDNF to mature BDNF in the hippocampus. J. Neurosci. 31, 12963-12971.
Mizoguchi H, Yamada K, Mouri A, Niwa M, Mizuno T, Noda Y, Nitta A, Itohara S, Banno Y, Nabeshima T. (2007a) Role of matrix metalloproteinase and tissue inhibitor of MMP in methamphetamine-induced behavioral sensitization and reward: implications for dopamine receptor down-regulation and dopamine release. J. Neurochem. 102, 1548-1560.
Mizoguchi H, Yamada K, Mouri A, Niwa M, Mizuno T, Noda Y, Nitta A, Itohara S, Banno Y, Nabeshima T. (2007b) Reduction of methamphetamine-induced sensitization and reward in matrix metalloproteinase-2 and-9-deficient mice. J. Neurochem. 100, 1579-1588.
Murase S., Lantz C. L., Kim E., Gupta N., Higgins R., Stopfer M., Hoffman D. A. and Quinlan E. M. (2015) Matrix Metalloproteinase-9 Regulates Neuronal Circuit Development and Excitability. Mol. Neurobiol.
Nagy V., Bozdagi O. and Huntley G. W. (2007) The extracellular protease matrix metalloproteinase-9 is activated by inhibitory avoidance learning and required for long-term memory. Learn. Mem. 14, 655-664.
Nagy, V., Bozdagi, O., Matynia, A., Balcerzyk, M., Okulski, P., Dzwonek, J., Costa, R.M., Silva, A.J., Kaczmarek, L., Huntley, G.W. (2006) Matrix metalloproteinase-9 is required for hippocampal late-phase long-term potentiation and memory. J. Neurosci. 26, 1923-1934.
Nishiyama J, Yasuda R. (2015) Biochemical Computation for Spine Structural Plasticity. Neuron. 87: 63-75.
Niedringhaus M., Chen X., Dzakpasu R. and Conant K. (2012) MMPs and soluble ICAM-5 increase neuronal excitability within in vitro networks of hippocampal neurons. PLoS One 7.
Niedringhaus M., Chen X., Conant K. and Dzakpasu R. (2013) Synaptic Potentiation Facilitates Memory-like Attractor Dynamics in Cultured In vitro Hippocampal Networks. PLoS One 8.
Nikolaev E., Tischmeyer W., Krug M., Matthies H., Kaczmarek L., (1991) c-fos protooncogene expression in rat hippocampus and entorhinal cortex following tetanic stimulation of the perforant path. Brain Res., 560: 346-349.
Nikolaev E., Kaminska B., Tischmeyer W., M., Matthies H., Kaczmarek L. (1992a) Induction of expression of genes encoding transcription factors in rat brain elicited by behavioral training. Brain Res. Bull., 28: 479-484.
Nikolaev E., Werka T., Kaczmarek L. (1992b) c-fos protooncogene expression in rat brain after long term training of two-way active avoidance reaction. Behav. Brain Res., 48: 91-94.
Okulski P., Jay T.M., Jaworski J., Duniec K., Dzwonek J., Konopacki F.A., Wilczynski G.M., Sánchez-Capeli A., Mallet J., Kaczmarek L., (2007) TIMP-1 abolishes MMP-9-dependent long-lasting long-term potentiation in the prefrontal cortex. Biol. Psychiatry 62, 359-363.
Paribello C., Tao L., Folino A., Berry-Kravis E., Tranfaglia M., Ethell I. M. and Ethell D. W. (2010) Open-label add-on treatment trial of minocycline in fragile X syndrome. BMC Neurol. 10, 91.
Peixoto R. T., Kunz P. A., Kwon H., Mabb A. M., Sabatini B. L., Philpot B. D. and Ehlers M. D. (2012) Transsynaptic signaling by activity-dependent cleavage of Neuroligin-1. Neuron 76, 667-667.
Reinhard S. M., Razak K. and Ethell I. (2015) A Delicate Balance: Role of MMP-9 in Brain Development and Pathophysiology of Neurodevelopmental Disorders. Front. Cell Neurosci. 9: 280.
Rivera S., Khrestchatisky M., Kaczmarek L., Rosenberg G. A. and Jaworski D. M. (2010) Metzincin proteases and their inhibitors: foes or friends in nervous system physiology? J. Neurosci. 30, 15337-15357.
Rotschafer S. E., Trujillo M. S., Dansie L. E., Ethell I. M. and Razak K. A. (2012) Minocycline treatment reverses ultrasonic vocalization production deficit in a mouse model of Fragile X Syndrome. Brain Res. 1439, 7-14.
Sala C. and Segal M. (2014) Dendritic spines: the locus of structural and functional plasticity. Physiol. Rev. 94, 141-188.
Samochowiec A., Grzywacz A., Kaczmarek L., Bienkowski P., Samochowiec J., Mierzejewski P., Preuss U. W., Grochans E. and Ciechanowicz A. (2010) Functional polymorphism of matrix metalloproteinase-9 (MMP-9) gene in alcohol dependence: family and case control study. Brain Res. 1327, 103-106.
Shinoe T., Goda Y. (2015) Tuning synapses by proteolytic remodeling of the adhesive surface. Curr Opin Neurobiol. 35, 148-155.
Sidhu H., Dansie L. E., Hickmott P. W., Ethell D. W. and Ethell I. M. (2014) Genetic removal of matrix metalloproteinase 9 rescues the symptoms of fragile X syndrome in a mouse model. J. Neurosci. 34, 9867-9879.
Siller S. S. and Broadie K. (2012) Matrix metalloproteinases and minocycline: therapeutic avenues for fragile X syndrome. Neural plast. 2012, 124548.
Smith A. C., Scofield M. D. and Kalivas P. W. (2015) The tetrapartite synapse: Extracellular matrix remodeling contributes to corticoaccumbens plasticity underlying drug addiction. Brain Res.
Smith A.C., Kupchik Y.M., Scofield M.D., Gipson C.D., Wiggins A., Thomas C.A., Kalivas P.W. (2014) Synaptic plasticity mediating cocaine relapse requires matrix metalloproteinases. Nat. Neurosci. 17, 1655-1657.
Soleman S, Filippov MA, Dityatev A, Fawcett JW. (2013) Targeting the neural extracellular matrix in neurological disorders. Neuroscience. 2013 253: 194-213.
Spolidoro M., Putignano E., Munafo C., Maffei L. and Pizzorusso T. (2012) Inhibition of matrix metalloproteinases prevents the potentiation of nondeprived-eye responses after monocular deprivation in juvenile rats. Cereb. Cortex 22, 725-734.
Stamenkovic V., Stamenkovic S, Jaworski T, Gawlak M, Jovanovic M, Jakovcevski I, Wilczynski GM, Kaczmarek L, Schachner M, Radenovic L, Andjus PR. The extracellular matrix glycoprotein Tenascin-C and matrix metalloproteinases modify cerebellar structural plasticity by exposure to an enriched environment brain structure and function. Brain Struct. Funct., in press.
Stawarski M., Stefaniuk M. and Wlodarczyk J. (2014a) Matrix metalloproteinase-9 involvement in the structural plasticity of dendritic spines. Front Neuroanat. 8, 68.
Stawarski M, Rutkowska-Wlodarczyk I, Zeug A, Bijata M, Madej H, Kaczmarek L, Wlodarczyk J. (2014b) Genetically encoded FRET-based biosensor for imaging MMP-9 activity. Biomaterials 35, 1402-1410.
Szepesi Z.,Bijata M., Ruszczycki B., Kaczmarek L., Wlodarczyk J. (2013) Matrix metalloproteinases regulate the formation of dendritic spine head protrusions during chemically induced long-term potentiation. PLoS One, 8: e63314.
Szepesi Z., Hosy E., Ruszczycki B., Bijata M., Pyskaty M., Bikbaev A., Heine M., Choquet D., Kaczmarek L., Wlodarczyk J. (2014) Synaptically released matrix metalloproteinase activity in control of structural plasticity and the cell surface distribution of GluA1-AMPA receptors. PLoS One 9, e98274.
Szklarczyk A., Kaczmarek L., Physiology of matrix MMPs and their tissue inhibitors in the brain. Biotech Int., 17: 15-18, 2005.
Szklarczyk A., Lapinska J., Rylski M., McKay R. D. and Kaczmarek L. (2002) Matrix metalloproteinase-9 undergoes expression and activation during dendritic remodeling in adult hippocampus. J. Neurosci. 22, 920-930.
Tamura H, Ishikawa Y, Shiosaka S. (2013) Does extracellular proteolysis control mammalian cognition? Rev Neurosci. 24: 365-374.
Tian L., Stefanidakis M, Ning L, Van Lint P, Nyman-Huttunen H, Libert C, Itohara S, Mishina M, Rauvala H, Gahmberg CG. (2007) Activation of NMDA receptors promotes dendritic spine development through MMP-mediated ICAM-5 cleavage. J. Cell Biol. 178, 687-700.
Tishmeyer W., Kaczmarek L., Strausss M., Jork R., Matthies H., Accumulation of c-fos mRNA in rat hippocampus during acquisition of a brightness discrimination. Behav. Neural Biol. 54: 165-171, 1990.
Tsien RY. (2013) Very long-term memories may be stored in the pattern of holes in the perineuronal net. Proc Natl Acad Sci USA. 110: 12456-12461.
Tsilibary E., Tzinia A., Radenovic L., Stamenkovic V., Lebitko T., Mucha M., Pawlak R., Frischknecht R. and Kaczmarek L. (2014) Neural ECM proteases in learning and synaptic plasticity. Prog. Brain Res. 214, 135-157.
Vafadari B, Salamian A, Kaczmarek L. (2016) MMP-9 in translation: from molecule to brain physiology, pathology and therapy. J Neurochem. , in press.
van der Kooij M. , Fantin M. , Rejmak E., Grosse J., Zanoletti O., Fournier C., Ganguly K., Kalita K., Kaczmarek L., Sandi C. (2014) Role for MMP-9 in stress-induced downregulation of nectin-3 in hippocampal CA1 and associated behavioural alterations. Nat. Commun. 5, 4995.
Vandooren J., Van den Steen P. E. and Opdenakker G. (2013) Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9): the next decade. Crit. Rev. Biochem. Mol. Biol. 48, 222-272.
Verslegers M., Lemmens K., Van Hove I. and Moons L. (2013) Matrix metalloproteinase-2 and -9 as promising benefactors in development, plasticity and repair of the nervous system. Prog. Neurobiol. 105, 60-78.
Wang X. B., Bozdagi O., Nikitczuk J. S., Zhai Z. W., Zhou Q. and Huntley G. W. (2008) Extracellular proteolysis by matrix metalloproteinase-9 drives dendritic spine enlargement and long-term potentiation coordinately. Proc. Natl. Acad. Sci. U. S. A. 105, 19520-19525.
Wilczynski G.M., Konopacki F.A., Wilczek E., Lasiecka Z., Gorlewicz A., Michaluk P., Wawrzyniak M., Malinowska M., Okulski P., Kolodziej L.R., Konopka W., Duniec K., Mioduszewska B., Nikolaev E., Walczak A., Owczarek D., Gorecki D.C., Zuschratter W., Ottersen O.P., Kaczmarek L. (2008) Important role of matrix metalloproteinase 9 in epileptogenesis. J. Cell. Biol. 180, 1021-1035.
Wojtowicz T. and Mozrzymas J. W. (2010) Late phase of long-term potentiation in the mossy fiber-CA3 hippocampal pathway is critically dependent on metalloproteinases activity. Hippocampus 20, 917-921.
Wright J. W., Brown T. E. and Harding J. W. (2007) Inhibition of hippocampal matrix metalloproteinase-3 and -9 disrupts spatial memory. Neural Plast. 2007, 73813.
Wright J. W., Meighan S. E., Murphy E. S., Holtfreter K. L., Davis C. J., Olson M. L., Benoist C. C., Muhunthan K. and Harding J. W. (2006) Habituation of the head-shake response induces changes in brain matrix metalloproteinases-3 (MMP-3) and -9. Behav. Brain Res. 174, 78-85.
Yong VW. (2005) Metalloproteinases: mediators of pathology and regeneration in the CNS. Nat. Rev. Neurosci. 6, 931-944.
Yong V. W., Krekoski C. A., Forsyth P. A., Bell R. and Edwards D. R. (1998) Matrix metalloproteinases and diseases of the CNS. Trends Neurosci. 21, 75-80.
Zagulska-Szymczak S, Filipkowski RK, Kaczmarek L. (2001) Kainate-induced genes in the ns from expression patterns. Neurochem Int. 38: 485-501.