Thyroid hormones (THs) are essential for the development and function of the central nervous system (CNS), not only for neuronal cells but also for glial development and differentiation. In adult CNS, both hypo- and hyper-thyroidism may affect psychological condition and potentially increase the risk of cognitive impairment and neurodegeneration including Alzheimer’s disease (AD). We have reported non-genomic effects of tri-iodothyronine (T3) on microglial functions and its signaling in vitro (MORI et al., 2015). Here we report the effects of hyperthyroidism on glial cells in vivo using young and old male and female mice. Immunohistochemical analyses showed glial activation are sex- and age-dependent. We also injected fluorescent-labeled amyloid β peptide (Aβ1-42) intracranially to L-thyroxine (T4)–injected hyperthyroid model mice and observed sex-dependent microglial phagocytosis in vivo as well. These results may partly explain the gender- and age-dependent differences in neurological and psychological symptoms of thyroid dysfunction.
Introduction
There is a close relationship between the endocrine system and the central nervous system (CNS). Among hormones closely related to the nervous system, thyroid hormones (THs) are critical for the development and function of the CNS (BERNAL, 2000, 2015; DI LIEGRO, 2008; GOMES et al., 2001; PORTERFIELD and HENDRICH, 1993; STENZEL and HUTTNER, 2013; ZOELLER and ROVET, 2004); not only for neuronal cells but also for glial cells (GARCIA-SEGURA et al., 1996; MOHACSIK et al., 2011), especially for their development and differentiation (BAXI et al., 2014; BILLON et al., 2001; DEZONNE et al., 2015; GOMES et al., 1999; JONES et al., 2003; LIMA et al., 2001).
L-thyroxine (T4) is the major TH secreted by the follicular cells of thyroid gland and taken up to astrocytes after transported into the brain through transporters (BERNAL, 2000; BERNAL et al., 2015; BRAUN et al., 2011; HENNEMANN et al., 2001; SCHWEIZER and KOHRLE, 2013; WIRTH et al., 2014). TH transport into astrocytes is also dependent on the H+ concentration inside the cells, involving a mechanism linked to the activity of the Na+-H+ exchanger (BESLIN et al., 1995). T4 in astrocytes is de-iodinated by type2-deiodinase (D2) to produce T3 (DI LIEGRO, 2008; GUADANO-FERRAZ et al., 1999; GUADANOFERRAZ et al., 1997). T3 is then transported out to the brain parenchyma (BERNAL et al., 2015), being apparently released from astrocytes.
Brain function depends on an intimate neuron to glia and glia to neuron signaling (DEZONNE et al., 2013; GOMES et al., 1999; MENDES-DE-AGUIAR et al., 2008; MORTE and BERNAL, 2014; NIEDERKINKHAUS et al., 2009). Thyroid hormone may have protective effects non-genomically or genomically on neurons and glial cells in the setting of acute brain ischemia (LIN et al., 2011). Therefore, any impairment of TH supply to the developing CNS causes severe and irreversible changes in the overall architecture and function of the human brain, leading to various neurological dysfunctions (DI LIEGRO, 2008; DUNTAS and BIONDI, 2013; HENRICHS et al., 2010). Although the importance of THs on development and various functions of CNS have been reported, precise actions of THs on glial cells have not yet been well explored. We previously investigated the effect of THs on primary cultured microglia and their intracellular signaling (MORI et al., 2015). T3-induced microglial migration and phagocytosis as well as morphological changes appeared to be non-genomic effect, which seem to be relevant to regulation of proliferation and motility of endothelial cells and certain tumor cells (P. J. DAVIS et al., 2016; P. J. DAVIS et al., 2008; KALYANARAMAN et al., 2014; LEONARD, 2008). There are cytoplasmic TH receptors (TRs) in addition to nuclear TRs; T3 might interact with a plasma-membrane-associated TH receptora variant (KALYANARAMAN et al., 2014), and with cytoplasmic TH receptor b (MARTIN et al., 2014), while T4 interacts with integrin avb3 (BERNAL, 2007; CHENG et al., 2010; P. J. DAVIS et al., 2016; P. J. DAVIS et al., 2008), though both nongenomic and genomic effects can overlap in the nucleus (P. J. DAVIS et al., 2016). In astrocyte T4 alters actin polymerization and iodothyronine deiodinase activity through non-genomic pathway, which may also contribute to the normal brain development through multiple signal transduction pathways (KOIBUCHI, 2013). In the present study, in addition to the analyses of TH action in cellular level, morphological and functional changes of glial cells in response to THs were analyzed in T4-injected animal, i.e. hyperthyroid mouse model.
Methods
Animals
The study was approved by the Animal Research Committee of Kyushu University and carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and experimental procedures were based on the Guidelines of the Committee for Animal Care and Use of Kyushu University. Hyperthyroidism was induced by intraperitoneally injecting T4 (0.3 mg/kg) 4 times during 2 weeks.
Immunohistochemical analysis
Mice were anesthetized by pentobarbital sodium (50 mg/kg, i.p.) and perfused transcardially with saline followed by 4% paraformaldehyde in 0.1M phosphate-buffered saline. The brain was removed, postfixed in the same fixative and placed in 20% sucrose solution for 24 h at 4 °C. Transverse brain sections (30 mm) were sliced by a HM 550 cryostat (Micro-edge Instruments Co., Tokyo, Japan), then incubated with rabbit anti-mouse Iba-1 IgG (1:2000; Wako) and mouse anti-mouse GFAP IgG (1:2000; Millipore) in 10% block ace for 18 h at 4 °C. The sections were incubated with Alexa fluor 488 conjugated goat anti-rabbit IgG (1:1000; Molecular Probes) and Alexa fluor 568 conjugated goat anti-mouse IgG (1:1000; Molecular Probes) for 3 hr at room temperature. Sections were mounted on coverslips with
PermaFluor Aqueous Mounting Medium (Thermo Scientific, Yokohama, Japan). The sections were analyzed using a fluorescence microscope (BZ9000, Keyence, Japan).
In vivo intracortical 5-FAM-labeled Ab1-42 delivery
Male/female C57/BL6J hyperthyroid model mice (8 weeks old) were anesthetized with pentobarbital (12.5 mg/kg) and placed in a stereotactic apparatus. A 2 ml Hamilton syringe with a 25 gauge needle was inserted into the right cortex through a small hole drilled through the skull, and then 2 ml of fluorescently labeled 5-FAM-labeled Ab1-42 (1 mg/ml) was injected.
For intracortical injection, the microsyringe was inserted into brain at the following coordinates; anterior, 0.6 mm; lateral, 1.6 mm; and dorsoventral, 0.8 mm. n = 3 - 4 animals were used per experimental group. 3 days interval after microinjection of Ab1-42, each mouse was anesthetized with pentobarbital (50 mg/kg, i.p.) and transcardially perfused with saline followed by 4% paraformaldehyde in 0.1M phosphate-buffered saline (PBS; 80 mM Na2HPO4, 20 mM KH2PO4, 150 mMNaCl, pH 7.4). The brain was removed, postfixed in the same fixative and cryoprotected for 24 h in 20% sucrose solution at 4 °C. Microglia in the sections containing lesion were immunohistochemically stained with rabbit anti-mouse Iba-1 IgG (1:2000; Wako) and Alexa fluor 568 conjugated goat anti-rabbit IgG (1:1000; Molecular Probes) as mentioned below. The sections were analysed by a fluorescence microscope (BZ9000, Keyence, Japan).
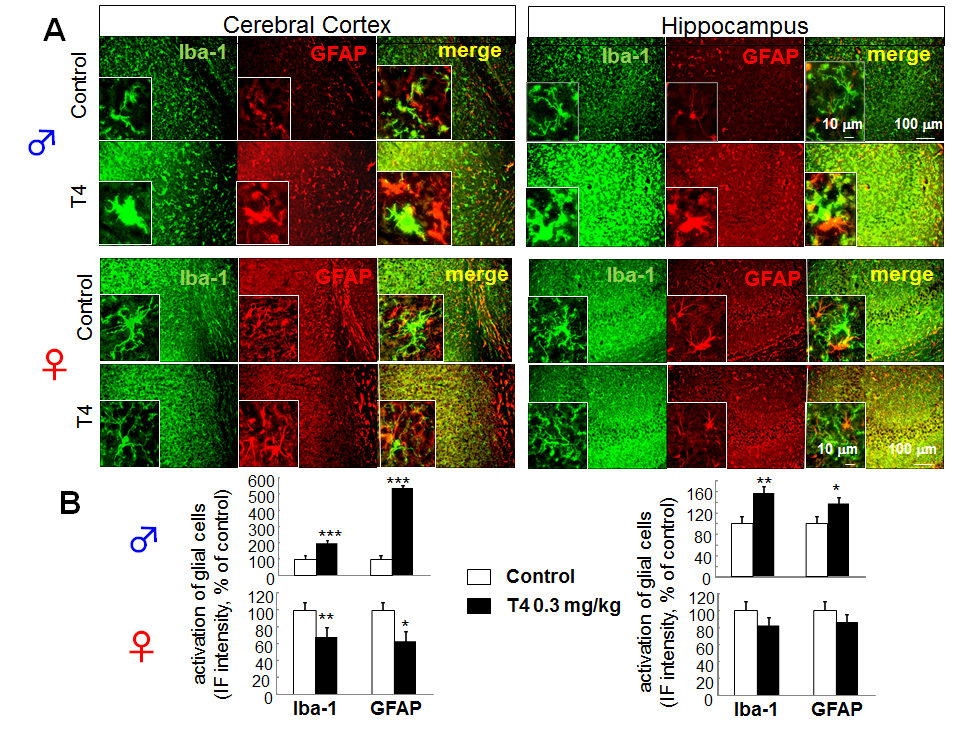
Figure 1. Sex-dependent glial activation in hyperthyroid model of young mice. A. Differential glial activation in cerebral cortex and hippocampus of hyperthyroid mouse model. Double immunofluorescence staining of Iba-1 and GFAP in slice preparation (30 μm) from cerebral cortex and hippocampus of T4-treated hyperthyroid model and control (vehicle-treated) mice in young (8 week old) male ( upper panels) and female ( lower panels). B. The relative fluorescence intensity of Iba-1 and GFAP immunoreactivity in cerebral cortex and hippocampus of young male (upper panels) and female (lower panels). *p<0.05, **p<0.01, ***p<0.005 vs control (Sheffe’s test)
Results
Both microglia and astrocytes were morphologically activated with abnormal level of THs. We made hyperthyroid model mice with young and old mice and observed the effects of hyperthyroidism on glial cells. Interestingly the effects of THs were sex- and age-dependent.
Sex-dependent effects of hyperthyroidism on microglial and astrocytic in cerebral cortex and hippocampus
To determine whether change in TH level affects microglia in vivo, we investigated the effects of hyperthyroidism on microglia and astrocytes in the cerebral cortex and hippocampus by immunohistochemical analysis. In the hyperthyroid model using young (8 week old) male mice, retraction of processes and enlargement of cell body of microglia and astrocytes were observed in cerebral cortex and hippocampus, especially in dentate gyrus (DG) and CA3 region (Fig. 1A). As a result, the Iba-1 and GFAP immunoreactivities were significantly increased in both regions (cortical immunoreactivity; 197.41 ± 16.86% of control for Iba-1, 534.38 ± 70.77% of control for GFAP) (hippocampal immunoreactivity; 155.43% ± 12.03 of control for Iba-1, 136.11 ± 11.11% of control for GFAP) (Fig. 1B, upper panels). This microglial and astrocytic activation was not observed in young (8 weeks old) female hyperthyroid mice (cortical immunoreactivity; 66.92 ± 5.68% of control for Iba-1, 62.20 ± 12.36% of control for GFAP) (hippocampal immunoreactivity; 81.61% ± 9.91 of control for Iba-1, 85.37 ± 9.98% of control for GFAP) (Fig. 1B, lower panels).
Age-dependent effects of hyperthyroidism on microglial and astrocytic activation in cerebral cortex and hippocampus
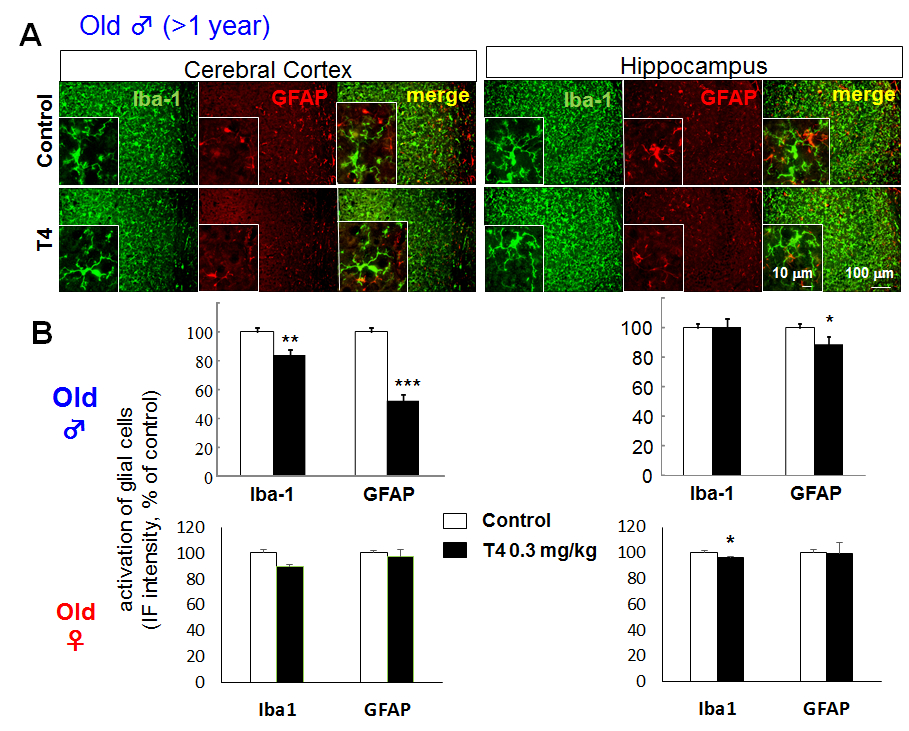
Though glial activation was observed in young male mice, the Iba-1 and GFAP immunoreactivities in cerebral cortex were decreased in old (>1 year old) male hyperthyroid mice (Fig. 2A), especially in posterior area of parietal association cortex (PTLp), primary somatosensory cortex (SSp), primary auditory cortex (AUDp) and dorsal/ventral part of auditory cortex (AUDd, AUDv). The cortical immunoreactivity was; 83.34% ± 4.26 of control for Iba-1, 52.43 ± 3.93% of control for GFAP. On the other hand, in hippocampus, only GFAP immunoreactivity, but not Iba-1 immunoreactivity, decreased significantly in old male mice (100.07 ± 5.71% of control for Iba-1, 88.22 ± 2.76% of control for GFAP) (Fig. 2B upper panels). In female mice, no apparent change was observed in both young and old mice, though statistically significant decrease in Iba-1 immunoreactivity in hippocampus was shown (Fig. 2B lower panels).
Figure 2. Glial activation in hyperthyroid model is not observed in aged mice even in male. A. Differential glial activation in cerebral cortex and hippocampus of T4-treated hyperthyroid model and control (vehicle-treated) mice in old (> 1 year old) male. There is no activation in glial cells in old male mice compared to young male mice (Fig. 1). B. The relative fluorescence intensity of Iba-1 and GFAP immunoreactivity in cerebral cortex and hippocampus of old male (upper panels) and female (lower panels). *p<0.05, **p<0.01, ***p<0.005 vs control (Sheffe’s test)
Sex-dependent effects of hyperthyroidism on microglial phagocytosis in vivo
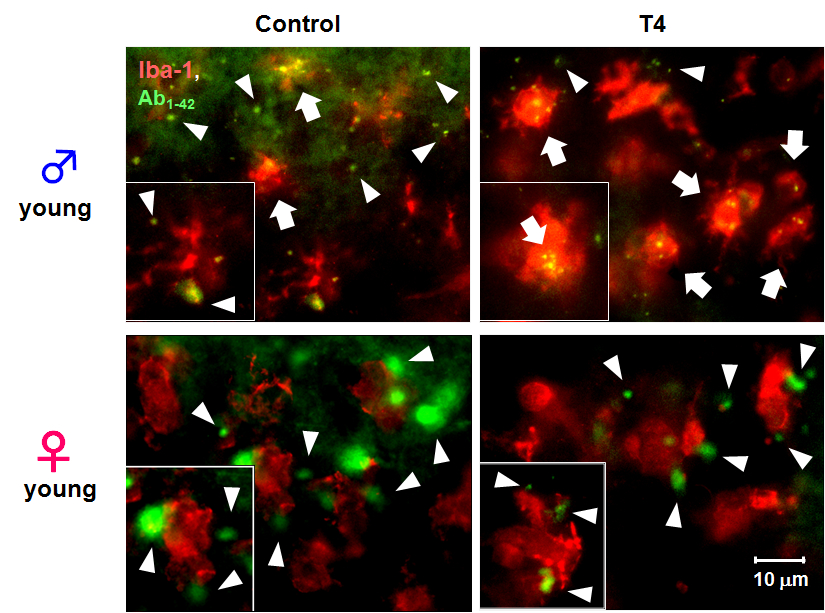
To examine the effect of THs and microglial activation in young male mice on microglial phagocytic function in vivo, we microinjected 5-FAM-labeled Ab1-42intracortically to control and hyperthyroid mice to observe microglial phagocytosis of Ab1-42in vivo. Phagocytosis of 5-FAM-labeled Ab1-42 by microglia was facilitated in young male hyperthyroid mice compared to the control group (Fig. 3, upper panels). However, this enhancement of microglial phagocytosis was not observed in young female hyperthyroid mice (Fig. 3, lower panels).
Figure 3. Sex-dependent microglial phagocytosis in hyperthyroidism in vivo. Typical images 5-FAM-labeled Aβ1-42 (green) and microglia (red, anti-Iba1) in cerebral cortex from control (vehicle-treated) or T4-treated hyperthyroid model mice in young male (upper panels) and female (lower panels). Extracellular fluorescent-labeled Aβ1-42 is observed as green (white arrow head), while phagocytosed Aβ1-42 within microglia can be observed as yellow particles (white arrow) (scale bar, 10 μm)
Discussion
Hyperthyroidism is an overactivation of thyroid gland, which lead to excessive production of THs. The prevalence of subclinical hyperthyroidism ranges from 1 to 15%, while subclinical hypothyroidism from 3 to 16% in individuals aged 60 years and older (BIONDI and COOPER, 2008). Even at subclinical level, hyperthyroidism in the elderly is suggested to increase the risk of cognitive decline, dementia and AD remarkably (KALMIJN et al., 2000; VAN OSCH et al., 2004; WIJSMAN et al., 2013). In addition, both hypothyroidism and hyperthyroidism, can cause psychiatric disorders such as schizophrenia, bipolar disorder, anxiety and depression. However, the role of microglia and astrocytes in this relationship between thyroid dysfunctions and neuropsychological disorders is largely unknown (NODA, 2015). Therefore, the effects of THs on microglia and astrocytes that act as major TH metabolism and local T3 production regulator were observed. The THs-sensitive regions of the brain are cerebral cortex and hippocampus (FONSECA et al., 2013; GUADANOFERRAZ et al., 1997). We made hyperthyroid mouse models using mice with different sex and age, then measured immunofluorescent intensity of Iba-1 and GFAP in each cerebral cortex and hippocampus as indicators of microglial and astrocytic activation.
In the present study, experimental hyperthyroidism of young (8 weeks old) male mice induced retraction of processes and enlargement of cell body of microglia and astrocytes in cerebral cortex and hippocampus (Fig. 1A), and significant increase in the Iba-1 and GFAP immunoreactivities (Fig. 1B). These results show that THs induce the morphological change and activation of microglia and astrocytes in vivo, as well as in vitro. Astrocyte is the major player of TH metabolism in the brain, which controls local T3 production by deiodination of T4. Therefore, this result suggests that elevated circulating and transported T4 stimulated brain astrocytes, might contribute to an increase in local brain T3 production, and provoked activation of microglia.
Contrary to what was observed in male, these morphological changes and activation of microglia and astrocytes were not observed in young (8 week old) female hyperthyroid mice (Fig. 1A and 1B, lower panels). Moreover, in old (>1 year old) male hyperthyroid mice, a significant decrease in Iba-1 and GFAP immunoreactivities in cerebral cortex was observed (Fig. 2A, 2B upper panels).
To examine whether this microglial activation induced by hyperthyroidism affect the microglial function in vivo, we performed intracortical microinjection of 5-FAM-labeled Ab1-42 to control and hyperthyroid mice, then observed microglial phagocytosis of Ab1-42 within the brain by immunohistochemical analysis. Microglial phagocytosis of 5-FAM-labeled Ab1- 42 was facilitated in young male hyperthyroid mice compared to the control group (Fig. 3, lower panels). However, similar to the results of glial activation, this enhancement of microglial phagocytosis was not observed in young female hyperthyroid mice (Fig. 3, lower panels). In aspect of sexual difference, it is reported that low and high thyrotropin levels were associated with an increased risk of AD in female but not in male (TAN et al., 2008). Therefore this differential activation of microglia and astrocytes in hyperthyroid model mice suggests that THs regulate their function through complex mechanisms, involving various factors, for example change in serum concentration of insulin-like growth factor II (TADA et al., 1994b) or apolipoproteins (TADA et al., 1994a) , in addition to sex- and age-dependent hormonal changes. Alternatively TH transporter expression might change with age or sex as reported in liver (ENGELS et al., 2015).
Age and/or sex characteristics of the thyroid hormone synthesis or function was reported decades ago (DAINAT et al., 1984; F. B. DAVIS et al., 1982; DUCASSOU et al., 1980;ERFURTH et al., 1984; EVERED et al., 1978; GEORGIEV and PETKOV, 1986; GRANDHI and BROWN, 1975; GREGERMAN, 1963; HANSEN et al., 1975; HEGEDUS et al., 1983; IVERSEN and PEDERSEN, 1979; KAPITOLA et al., 1969; LOSKUTOVA, 1974; NAKAI et al., 1981; SEGAL et al., 1982; TENORE et al., 1980; TROUT, 1974; WENZEL et al., 1974; WILKE, 1983; WILSON and JOHNSON, 1964; YOUSEF and LUICK, 1971). Gender dependence in the hormone content, including T3, of the immune cells (CSABA and PALLINGER, 2009) and sex dependence of autoimmune disease (ESTIENNE et al., 2002) were also reported, while no significant differences were observed for prolactin, free T3 and free T4 between the 21-30-year age group and the > 70-year age group (ELMLINGER et al., 2003). Since thyroid dysfunction increases with age, changes in THs levels in the elderly could be a factor affecting the development of neurodegenerative diseases including Alzheimer's disease (VILLANUEVA et al., 2013). Nevertheless, differential activation of glial cells in the brain has been totally unknown. The current findings will give us a hint to understand the molecular basis of the gender- and age-dependent phenomena due to TH abnormalities.
Age-dependent phenomena are also reported for the effect of neurotransmitter. For example, glutamate-dependent neuroglial calcium signaling differs between young and adult brain. It is because astrocytic expression of mGluR5 is developmentally regulated and is undetectable after postnatal week 3 (SUN et al., 2013). Age-specific localization of NMDA receptors on oligodendrocytes and age-dependent process in ischemic injury to white matter are also reported (BALTAN, 2009, 2014, 2015; BALTAN et al., 2008). Overall, we may need to highlight the importance of age and gender for better understanding of neurological disorders and in any successful therapy.
| Attachment | Size |
|---|---|
| 1.23 MB |
We thank Research Support Center, Graduate School of Medical Sciences, Kyushu University for their experimental help.
BALTAN S. (2009). Ischemic injury to white matter: an age-dependent process. Neuroscientist,15(2), 126-133. doi: 10.1177/1073858408324788
BALTAN S. (2014). Excitotoxicity and mitochondrial dysfunction underlie age-dependent ischemic white matter injury. AdvNeurobiol,11, 151-170. doi: 10.1007/978-3-319-08894-5_8
BALTAN S. (2015). Age-specific localization of NMDA receptors on oligodendrocytes dictates axon function recovery after ischemia. Neuropharmacology. doi: 10.1016/j.neuropharm.2015.09.015
BALTAN S., BESANCON E. F., MBOW B., YE Z., HAMNER M. A., and RANSOM B. R. (2008). White matter vulnerability to ischemic injury increases with age because of enhanced excitotoxicity. J Neurosci,28(6), 1479-1489. doi: 10.1523/JNEUROSCI.5137-07.2008
BAXI E. G., SCHOTT J. T., FAIRCHILD A. N., KIRBY L. A., KARANI R., UAPINYOYING P., et al. (2014). A selective thyroid hormone beta receptor agonist enhances human and rodent oligodendrocyte differentiation. Glia,62(9), 1513-1529. doi: 10.1002/glia.22697
BERNAL J. (2000). Thyroid Hormones in Brain Development and Function. In L. J. De Groot, P. Beck-Peccoz, G. Chrousos, K. Dungan, A. Grossman, J. M. Hershman, C. Koch, R. McLachlan, M. New, R. Rebar, F. Singer, A. Vinik& M. O. Weickert (Eds.), Endotext. South Dartmouth (MA).
BERNAL J. (2007). Thyroid hormone receptors in brain development and function. Nat ClinPractEndocrinolMetab, 3(3), 249-259. doi: 10.1038/ncpendmet0424
BERNAL J. (2015). Thyroid Hormones in Brain Development and Function. Endotext (Internet)In: De Groot LJ, Beck-Peccoz P, Chrousos G, Dungan K, Grossman A, Hershman JM, Koch C, McLachlan R, New M, Rebar R, Singer F, Vinik A, Weickert MO, editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-2015 Sep 2.
BERNAL J., GUADANO-FERRAZ A., and MORTE B. (2015). Thyroid hormone transporters-- functions and clinical implications. Nat Rev Endocrinol,11(7), 406-417. doi: 10.1038/nrendo.2015.66
BESLIN A., CHANTOUX F., BLONDEAU J. P., and FRANCON J. (1995). Relationship between the thyroid hormone transport system and the Na(+)-H+ exchanger in cultured rat brain astrocytes. Endocrinology,136(12), 5385-5390. doi: 10.1210/endo.136.12.7588286
BILLON N., TOKUMOTO Y., FORREST D., and RAFF M. (2001). Role of thyroid hormone receptors in timing oligodendrocyte differentiation. Dev Biol,235(1), 110-120. doi: 10.1006/dbio.2001.0293
BIONDI B., and COOPER D. S. (2008). The clinical significance of subclinical thyroid dysfunction. Endocr Rev, 29(1), 76-131. doi: 10.1210/er.2006-0043
BRAUN D., KINNE A., BRAUER A. U., SAPIN R., KLEIN M. O., KOHRLE J., et al. (2011). Developmental and cell type-specific expression of thyroid hormone transporters in the mouse brain and in primary brain cells. Glia,59(3), 463-471. doi: 10.1002/glia.21116
CHENG S. Y., LEONARD J. L., and DAVIS P. J. (2010). Molecular aspects of thyroid hormone actions. Endocr Rev,31(2), 139-170. doi: 10.1210/er.2009-0007
CSABA G., and PALLINGER E. (2009). Gender dependence in the hormone content of the immune cells. ActaPhysiol Hung,96(1), 45-50. doi: 10.1556/APhysiol.96.2009.1.5
DAINAT J., BRESSOT C., BACOU F., REBIERE A., and VIGNERON P. (1984). Perinatal age and sex variations of the triiodothyronine nuclear receptors in the chick pectoralis major muscle. Mol Cell Endocrinol,35(2-3), 215-220.
DAVIS F. B., SPECTOR D. A., DAVIS P. J., HIRSCH B. R., WALSHE J. J., and YOSHIDA K. (1982). Comparison of pituitary-thyroid function in patients with endstage renal disease and in age- and sex-matched controls. Kidney Int,21(2), 362-364.
DAVIS P. J., GOGLIA F., and LEONARD J. L. (2016). Nongenomic actions of thyroid hormone. Nat Rev Endocrinol, 12(2), 111-121. doi: 10.1038/nrendo.2015.205
DAVIS P. J., LEONARD J. L., and DAVIS F. B. (2008). Mechanisms of nongenomic actions of thyroid hormone. Front Neuroendocrinol,29(2), 211-218. doi: 10.1016/j.yfrne.2007.09.003
DEZONNE R. S., LIMA F. R., TRENTIN A. G., and GOMES F. C. (2015). Thyroid hormone and astroglia: endocrine control of the neural environment. J Neuroendocrinol. doi: 10.1111/jne.12283
DEZONNE R. S., STIPURSKY J., ARAUJO A. P., NONES J., PAVAO M. S., PORCIONATTO M., et al. (2013). Thyroid hormone treated astrocytes induce maturation of cerebral cortical neurons through modulation of proteoglycan levels. Front Cell Neurosci,7, 125. doi: 10.3389/fncel.2013.00125
DI LIEGRO I. (2008). Thyroid hormones and the central nervous system of mammals (Review).Mol Med Report, 1(3), 279-295.
DUCASSOU D., RASHEDI M., BRENDEL A., and GASPAROUX S. (1980). [Thyroxin binding globulin (TBG) radioimmunoassay. Normal patients results according to age, and sex, and in the cases of dysthyroidisms (author's transl)]. PatholBiol (Paris),28(3), 168-172.
DUNTAS L. H., and BIONDI B. (2013). The interconnections between obesity, thyroid function, and autoimmunity: the multifold role of leptin. Thyroid,23(6), 646-653. doi: 10.1089/thy.2011.0499
ELMLINGER M. W., DENGLER T., WEINSTOCK C., and KUEHNEL W. (2003). Endocrine alterations in the aging male. ClinChem Lab Med,41(7), 934-941. doi: 10.1515/CCLM.2003.142
ENGELS K., RAKOV H., ZWANZIGER D., MOELLER L. C., HOMUTH G., KOHRLE J., et al. (2015). Differences in Mouse Hepatic Thyroid Hormone Transporter Expression with Age and Hyperthyroidism. Eur Thyroid J,4(Suppl 1), 81-86. doi: 10.1159/000381020
ERFURTH E. M., NORDEN N. E., HEDNER P., NILSSON A., and EK L. (1984). Normal reference interval for thyrotropin response to thyroliberin: dependence on age, sex, free thyroxin index, and basal concentrations of thyrotropin. ClinChem,30(2), 196-199.
ESTIENNE V., DUTHOIT C., REICHERT M., PRAETOR A., CARAYON P., HUNZIKER W., et al. (2002). Androgen-dependent expression of FcgammaRIIB2 by thyrocytes from patients with autoimmune Graves' disease: a possible molecular clue for sex dependence of autoimmune disease. FASEB J,16(9), 1087-1092. doi: 10.1096/fj.01-0998hyp
EVERED D. C., TUNBRIDGE W. M., HALL R., APPLETON D., BREWIS M., CLARK F., et al. (1978). Thyroid hormone concentrations in a large scale community survey. Effect of age, sex, illness and medication. ClinChimActa,83(3), 223-229.
FONSECA T. L., CORREA-MEDINA M., CAMPOS M. P. O., WITTMANN G., WERNECK-DE-CASTRO J. P., DRIGO R. A. E., et al. (2013). Coordination of hypothalamic and pituitary T3 production regulates TSH expression. Journal of Clinical Investigation,123(4), 1492-1500. doi: Doi 10.1172/Jci61231
GARCIA-SEGURA L. M., CHOWEN J. A., and NAFTOLIN F. (1996). Endocrine glia: roles of glial cells in the brain actions of steroid and thyroid hormones and in the regulation of hormone secretion. Front Neuroendocrinol,17(2), 180-211. doi: 10.1006/frne.1996.0005
GEORGIEV P., and PETKOV P. I. (1986). [Age and sex characteristics of the level of thyroid hormone synthesis in the Camborough hybrid]. Vet Med Nauki,23(2), 43-47.
GOMES F. C., LIMA F. R., TRENTIN A. G., and MOURA NETO V. (2001). Thyroid hormone role in nervous system morphogenesis. Prog Brain Res,132, 41-50. doi: 10.1016/S0079-6123(01)32064-2
GOMES F. C., MAIA C. G., DE MENEZES J. R., and NETO V. M. (1999). Cerebellar astrocytes treated by thyroid hormone modulate neuronal proliferation. Glia,25(3), 247-255.
GRANDHI R. R., and BROWN R. G. (1975). Thyroid metabolism in the recessive sex-linked dwarf female chicken. 1. Age related changes in thyroid hormone synthesis and circulating thyroid hormone levels. PoultSci,54(2), 488-493.
GREGERMAN R. I. (1963). Estimation of thyroxine secretion rate in the rat by the radioactive thyroxine turnover technique: influences of age, sex and exposure to cold. Endocrinology,72, 382-392. doi: 10.1210/endo-72-3-382
GUADANO-FERRAZ A., ESCAMEZ M. J., RAUSELL E., and BERNAL J. (1999). Expression of type 2 iodothyronine deiodinase in hypothyroid rat brain indicates an important role of thyroid hormone in the development of specific primary sensory systems. J Neurosci,19(9), 3430-3439.
GUADANOFERRAZ A., OBREGON M. J., STGERMAIN D. L., and BERNAL J. (1997). The type 2 iodothyronine deiodinase is expressed primarily in glial cells in the neonatal rat brain.Proceedings of the National Academy of Sciences of the United States of America, 94(19),10391-10396. doi: DOI 10.1073/pnas.94.19.10391
HANSEN J. M., SKOVSTED L., and SIERSBAEK-NIELSEN K. (1975). Age dependent changes in iodine metabolism and thyroid function. ActaEndocrinol (Copenh),79(1), 60-65.
HEGEDUS L., PERRILD H., POULSEN L. R., ANDERSEN J. R., HOLM B., SCHNOHR P., et al. (1983). The determination of thyroid volume by ultrasound and its relationship to body weight, age, and sex in normal subjects. J ClinEndocrinolMetab,56(2), 260-263. doi: 10.1210/jcem-56-2-260
HENNEMANN G., DOCTER R., FRIESEMA E. C., DE JONG M., KRENNING E. P., and VISSER T. J. (2001). Plasma membrane transport of thyroid hormones and its role in thyroid hormone metabolism and bioavailability. Endocr Rev,22(4), 451-476. doi: 10.1210/edrv.22.4.0435
HENRICHS J., BONGERS-SCHOKKING J. J., SCHENK J. J., GHASSABIAN A., SCHMIDT H. G., VISSER T. J., et al. (2010). Maternal thyroid function during early pregnancy and cognitive functioning in early childhood: the generation R study. J ClinEndocrinolMetab,95(9), 4227-4234. doi: 10.1210/jc.2010-0415
IVERSEN E., and PEDERSEN K. O. (1979). Unconjugated thyroxine and triiodothyronine in urine: influence of age, sex, drugs and thyroid function. Scand J Clin Lab Invest,39(1), 7-13.
JONES S. A., JOLSON D. M., CUTA K. K., MARIASH C. N., and ANDERSON G. W. (2003). Triiodothyronine is a survival factor for developing oligodendrocytes. Mol Cell Endocrinol,199(1-2), 49-60.
KALMIJN S., MEHTA K. M., POLS H. A., HOFMAN A., DREXHAGE H. A., and BRETELER M. M. (2000). Subclinical hyperthyroidism and the risk of dementia. The Rotterdam study.ClinEndocrinol (Oxf), 53(6), 733-737.
KALYANARAMAN H., SCHWAPPACHER R., JOSHUA J., ZHUANG S., SCOTT B. T., KLOS M., et al. (2014). Nongenomic thyroid hormone signaling occurs through a plasma membrane-localized receptor. Sci Signal,7(326), ra48. doi: 10.1126/scisignal.2004911
KAPITOLA J., SCHULLEROVA M., and SCHREIBEROVA O. (1969). [The action of thyrotropin on blood flow and radioiodine accumulation in the rat thyroid gland: the effect of age, sex and blockade with dried thyroid]. Sb Lek,71(10), 283-288.
KOIBUCHI N. (2013). The role of thyroid hormone on functional organization in the cerebellum. Cerebellum, 12(3), 304-306. doi: 10.1007/s12311-012-0437-8
LEONARD J. L. (2008). Non-genomic actions of thyroid hormone in brain development. Steroids,73(9-10), 1008-1012. doi: 10.1016/j.steroids.2007.12.016
LIMA F. R., GERVAIS A., COLIN C., IZEMBART M., NETO V. M., and MALLAT M. (2001). Regulation of microglial development: a novel role for thyroid hormone. J Neurosci,21(6), 2028-2038.
LIN H. Y., DAVIS F. B., LUIDENS M. K., MOUSA S. A., CAO J. H., ZHOU M., et al. (2011). Molecular basis for certain neuroprotective effects of thyroid hormone. Front MolNeurosci,4, 29. doi: 10.3389/fnmol.2011.00029
LOSKUTOVA E. A. (1974). [State of the thyroid gland in female rats of different age after ovariectomy and administration of sex hormones]. ProblEndokrinol (Mosk),20(5), 65-69.
MARTIN N. P., MARRON FERNANDEZ DE VELASCO E., MIZUNO F., SCAPPINI E. L., GLOSS B., ERXLEBEN C., et al. (2014). A rapid cytoplasmic mechanism for PI3 kinase regulation by the nuclear thyroid hormone receptor, TRbeta, and genetic evidence for its role in the maturation of mouse hippocampal synapses in vivo. Endocrinology,155(9), 3713-3724. doi: 10.1210/en.2013-2058
MENDES-DE-AGUIAR C. B., ALCHINI R., DECKER H., ALVAREZ-SILVA M., TASCA C. I., and TRENTIN A. G. (2008). Thyroid hormone increases astrocytic glutamate uptake and protects astrocytes and neurons against glutamate toxicity. J Neurosci Res,86(14), 3117-3125. doi: 10.1002/jnr.21755
MOHACSIK P., ZEOLD A., BIANCO A. C., and GEREBEN B. (2011). Thyroid hormone and the neuroglia: both source and target. J Thyroid Res,2011, 215718. doi: 10.4061/2011/215718
MORI Y., TOMONAGA D., KALASHNIKOVA A., FURUYA F., AKIMOTO N., IFUKU M., et al. (2015). Effects of 3, 3’, 5-triiodothyronine on microglial functions. Glia,in press.
MORTE B., and BERNAL J. (2014). Thyroid hormone action: astrocyte-neuron communication. Front Endocrinol (Lausanne), 5, 82. doi: 10.3389/fendo.2014.00082
NAKAI R., OKANO K., and HARASAWA M. (1981). [Effects of age and sex on serum thyroid hormone levels in normal human subjects (author's transl)]. Nihon Ronen IgakkaiZasshi,18(6), 417-424.
NIEDERKINKHAUS V., MARX R., HOFFMANN G., and DIETZEL I. D. (2009). Thyroid hormone (T3)-induced up-regulation of voltage-activated sodium current in cultured postnatal hippocampal neurons requires secretion of soluble factors from glial cells. MolEndocrinol, 23(9), 1494-1504. doi: 10.1210/me.2009-0132
NODA M. (2015). Possible role of glial cells in the relationship between thyroid dysfunction and mental disorders. Front Cell Neurosci,9, 194. doi: 10.3389/fncel.2015.00194
PORTERFIELD S. P., and HENDRICH C. E. (1993). The role of thyroid hormones in prenatal and neonatal neurological development--current perspectives. Endocr Rev,14(1), 94-106.
SCHWEIZER U., and KOHRLE J. (2013). Function of thyroid hormone transporters in the central nervous system. BiochimBiophysActa,1830(7), 3965-3973. doi: 10.1016/j.bbagen.2012.07.015
SEGAL J., TROEN B. R., and INGBAR S. H. (1982). Influence of age and sex on the concentrations of thyroid hormone in serum in the rat. J Endocrinol,93(2), 177-181.
STENZEL D., and HUTTNER W. B. (2013). Role of maternal thyroid hormones in the developing neocortex and during human evolution. Front Neuroanat,7, 19. doi: 10.3389/fnana.2013.00019
SUN W., MCCONNELL E., PARE J. F., XU Q., CHEN M., PENG W., et al. (2013). Glutamate - dependent neuroglial calcium signaling differs between young and adult brain. Science,339(6116), 197-200. doi: 10.1126/science.1226740
TADA H., IRIE Y., YAGORO A., OHYA H., HAYASHI S., FUSHIMI R., et al. (1994a). Serum concentrations of apolipoproteins in patients with thyroid dysfunction. Thyroidology,6(3), 93-97.
TADA H., WATANABE Y., FUTAKUCHI Y., and AMINO N. (1994b). Change in serum concentration of insulin-like growth factor II (IGF-II) in patients with thyroid disease. Endocr J, 41(5), 541-545.
TAN Z. S., BEISER A., VASAN R. S., AU R., AUERBACH S., KIEL D. P., et al. (2008). Thyroid function and the risk of Alzheimer disease: the Framingham Study. Arch Intern Med,168(14), 1514-1520. doi: 10.1001/archinte.168.14.1514
TENORE A., OBERKOTTER L. V., and KOLDOVSKY O. (1980). Age- and sex-associated differences in the relationship of serum hexosaminidase and T4 levels in human neonates.Early Hum Dev, 4(1), 41-49.
TROUT E. C., JR. (1974). Effects of age and sex on the induction by triiodothyronine of cortisone delta4-5alpha-reductase and glucose 6-phosphate dehydrogenase of rat liver and adrenal. Steroids, 23(1), 133-144.
VAN OSCH L. A., HOGERVORST E., COMBRINCK M., and SMITH A. D. (2004). Low thyroid-stimulating hormone as an independent risk factor for Alzheimer disease. Neurology,62(11), 1967-1971.
VILLANUEVA I., ALVA-SANCHEZ C., and PACHECO-ROSADO J. (2013). The role of thyroid hormones as inductors of oxidative stress and neurodegeneration. Oxid Med Cell Longev,2013, 218145. doi: 10.1155/2013/218145
WENZEL K. W., MEINHOLD H., HERPICH M., ADLKOFER F., and SCHLEUSENER H. (1974). [TRH stimulation test with age and sex specific TSH response in normal subjects (authors' transl)]. KlinWochenschr,52(15), 722-727.
WIJSMAN L. W., DE CRAEN A. J., TROMPET S., GUSSEKLOO J., STOTT D. J., RODONDI N., et al. (2013). Subclinical thyroid dysfunction and cognitive decline in old age. PLoS One,8(3), e59199. doi: 10.1371/journal.pone.0059199
WILKE T. J. (1983). Influence of age and sex on the concentration of free thyroxin in serum and on the free thyroxin: total thyroxin ratio. ClinChem,29(7), 1428-1430.
WILSON W. P., and JOHNSON J. E. (1964). Thyroid Hormone and Brain Function. I. The Eeg in Hyperthyroidism with Observations on the Effect of Age, Sex, and Reserpine in the Production of Abnormalities. ElectroencephalogrClinNeurophysiol,16, 321-328.
WIRTH E. K., SCHWEIZER U., and KOHRLE J. (2014). Transport of thyroid hormone in brain. Front Endocrinol (Lausanne), 5, 98. doi: 10.3389/fendo.2014.00098
YOUSEF M. K., and LUICK J. R. (1971). Estimation of thyroxine secretion rate in reindeer, Rangifer tarandus: effects of sex, age and season. Comp BiochemPhysiol A Comp Physiol,40(3), 789-795.
ZOELLER R. T., and ROVET J. (2004). Timing of thyroid hormone action in the developing brain: clinical observations and experimental findings. J Neuroendocrinol,16(10), 809-818. doi: 10.1111/j.1365-2826.2004.01243.x